Radioactivity
# Radioactivity:- It is the phenomenon of spontaneous disintegration of the nucleus of an atom with the emission of one or more penetrating radiation like ∝ particles, β particle or γ particles.
The substance which spontaneously emit penetrating radiation were called radioactive substances. For example: Uranium, Colonium, Radium, Thorium, Actinium, etc
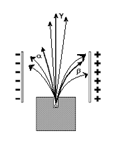
Becquerel Rays (Electrical nature of Radioactive Radiation)
In both the cases the narrow beam splits into three components.
1. The component which bend towards the left consist of positively charged particle called ∝-Rays.
2. The component which bend towards right consist of negatively charged particles called β-Rays.
3. The components which goes straight consist of neutral phonons are called γ-Rays.
# Laws of Radio-active disintegration:
Rutherford & Soddy Fazans give the laws of radioactive disintegration substances. This law states that -->
1. The process of radioactive disintegration is completely spontaneous is which the small amount of energy is emitted.
2. The emission of ∝, β, γ rays does not occur simultaneously from the nucleus, it means that at one time ∝, β, γ rays are emitted.
3. During ∝ decay when one element changes into another element the mass number of the new element decreases by 4 & its atomic number by 2. It is given by
4. During β-decay, the atomic number of the daughter atom increases by 1 & mass number remains same.
Very Very Important
The note of disintegration of a radioactive substance is directly proportional to the number of atom present initially at the sample of the radioactive substance, According to radioactive law
(dN/dt) ∝ N
(dN/dT) = (-λ) N (-because of disintegration)
Where λ is called decay constant
(dN/N) = (-λ) dt
Integrating both sides we have
= ∫(dN/N) = -λ ∫dt
= loge N = -λt + C —(1)
(C is integration constant)
When t = 0, then number of atom N = No, then equation
(1) becomes
loge No = -λ (0) + C
C = loge No —(2)
From equation (1) & (2)
loge N = -λt + loge No
-λt = loge N - loge No
loge N = loge No = -λt
loge(N/No) = -λt
∴ (log m – log n) = log (M/n)
loge (N/No) = -λt
N/No = e-λt
N = No e-λt
This equation represents, the radioactive decay law
==> it gives the number of active nuclei left after time t thus the number of active nuclei in a radioactive substance decreases exponentially with time.
# Half life of Radioactive substance
It is defined as the time is which any radioactive substance after disintegration becomes half of the original quantity. Let No is equal to number of radioactive nuclei present in the radioactive same initially at t = 0.
N is equal to number of radioactive nuclei left at any instant i.e. When t = T1/2, N = No/2
Now,
N = No e-λt
Where λ is radioactive decay constant putting above values in this equation.
No/2 = No e-λt
Taking logarithm side the me have
λ. T1/2 log ee = log e2
λ. T1/2 = log e2
T1/2 = log e2/λ
==> T1/2 = 2.303 log 102/λ
==> T1/2 = 2.303 x 0.3010/λ
T1/2 = 0.623/λ
Thus half life of λ radioactive substance is inversely ∝ to its decay constant & is independent of the number of No the number of radioactive nuclei present initially in the sample.
 SureDen
SureDen