Magnetic dipole moment of a revolving electron
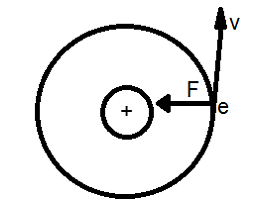
According to Bohr model of Hydrogen like atoms, negatively charged electron revolves around the positively charged nucleus. This uniform circular motion of the electron is equivalent to current loop which possess the magnetic dipole moment = IA
According to Bohr model of Hydrogen like atoms, negatively charged electron revolves around the positively charged nucleus. This uniform circular motion of the electron is equivalent to current loop which possess the magnetic dipole moment = IA
And
Current is I = e/t = e/(2πr/v) = ev/2πr
A = πr2 is the area of the current loop.
Then, the orbital magnetic moment of the electron is μI = IA = (ev/2πr) πr2
μI = evr/2 ----------------------(1)
Also, angular momentum of the electron is
L = Mevr ---------------- (2)
Dividing equation 1 by 2, we get
μI/1 =evr/(2/Mevr) = e/2Me
This is called gyromagnetic ratio and its value is 8.8 * 1010Ckg-1.
According to Bohr’s quantization condition, angular momentum
L = nh/2π
∴ μI = neh/4πMe
This equation gives orbital magnetic moment of an electron in nth orbit.
Bohr magnetron: It is defined as the magnetic moment associated with an electron due to its orbital motion in the first orbit of hydrogen atom. Then
μB = μI = eh/4πMe
Then,
μB = 9.27 * 10-24 Am2
And
Current is I = e/t = e/(2πr/v) = ev/2πr
A = πr2 is the area of the current loop.
Then, the orbital magnetic moment of the electron is μI = IA = (ev/2πr) πr2
μI = evr/2 ----------------------(1)
Also, angular momentum of the electron is
L = Mevr ---------------- (2)
Dividing equation 1 by 2, we get
μI/1 =evr/(2/Mevr) = e/2Me
This is called gyromagnetic ratio and its value is 8.8 * 1010Ckg-1.
According to Bohr’s quantization condition, angular momentum
L = nh/2π
∴ μI = neh/4πMe
This equation gives orbital magnetic moment of an electron in nth orbit.
Bohr magnetron: It is defined as the magnetic moment associated with an electron due to its orbital motion in the first orbit of hydrogen atom. Then
μB = μI = eh/4πMe
Then,
μB = 9.27 * 10-24 Am2
 SureDen
SureDen