Dielectrics
Dielectric:
A dielectric is a substance which does not allow the flow of charges though it but permits them to exert electrostatics forces on one another through it.
Examples: Wax, glass, Water, air, wood, rubber, stone, plastic etc.
Polar Dielectrics:
A molecules in which centre of mass of positive charges does not coincide with the centre of mass of negative charges is called polar dielectric. Ex. HCl, NH3, CO etc.
Non- polar dielectrics: A molecules in which the centre of mass of positive charges coincide with the centre of mass of negative charges is called non-polar molecule. They have normally zero dipole moment.
Example: H2, N2, O2, CO2, CH4, etc.
Polarization of a non-polar and polar dielectric in an external field:
Both polar and non-polar dielectrics develop a net dipole moment in the presence of an external field. This fact is called polarization of the dielectrics.
The Polarisation is defined as the dipole moment per unit volume and its magnitude is usually referred to as the dipole moment per unit volume and its magnitude is usually referred to as the polarization density. The direction of
is same as that of the external field E0.
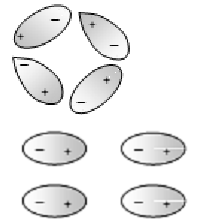
If E=0 dipoles align randomly in a polar dielectric
If E≠0 dipoles align in following fashion in a polar dielectric
Reduction of electric field by the polarization of a dielectric:
In case of a homogeneous and isotropic dielectric, the induced surface charges set up an electric field
(field due to polarization) inside the dielectric in a direction opposite to that of the external field
thus tend to reduce the original field in the dielectric. The resultant field E in the dielectric field will be equal to
Eo -Ep and directed in the direction of Eo .
The ratio of original filed and reduced field is known as dielectric constant or relative permittivity
Polarization density:
The induced dipole moment of dielectric per unit volume of dielectric is known as polarization density and is given by
Hence the polarization density may be defined as the charge induced per unit surface area.
Electric susceptibility:
If the field E is not large, then the polarization P is proportional to the resultant field E existing in the dielectric, i.e.
Where X is the proportionality constant called electric susceptibility. The multiplication factor ɛ0 is used to keep 𝜒 dimensionless.
Clearly,
Thus the ratio of the polarization to ɛ0 times the dielectric field is called electric susceptibility of the dielectric.
Relation between K and 𝜒:
The net electric field in a polarization dielectric is
Dividing both sides by E, we get
1 = K – 𝜒
K = 1 + 𝜒
Dielectric strength:
The maximum electric field that can exist in a dielectric without causing the breakdown of its insulating property is called dielectric strength of the material. The unit of dielectric strength is same as that of electric field V/m.
And the more common practical unit is kV mm-1
 SureDen
SureDen