Dipole Moment
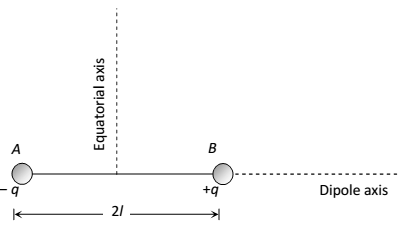
Electric Dipole: A pair of equal and opposite charges separate by a small distance is called electric dipole.
Dipole Moment: It measures the strength of an eclectic dipole whose magnitude is either charge times the separation between two opposite charges and the direction is along the dipole axis for the –ve to +ve charge.
Dipole Moment = either charge * a vector drawn from negative to positive charge , the SI unit is coulomb meter.
Example:
Dipoles are common in nature. In molecules like H2O , HCL , C2H5OH CH3COOH etc. the centre of positive of positive charges does not fall exactly over the centre of negative charges. Such molecules are called electric dipoles. They have permanent dipole moment.
Ideal or point dipole:
The dipole whose size 2a →0 and charge q →∞ in such a way that the dipole moment, p=q 2a has a finite value.
Dipole associated with individual atoms or molecules may be treated as ideal dipoles.
 SureDen
SureDen