Davisson & Germer Experiment
Experimental Verification of De-Broglie waves:
The wave nature of a moving particle is explained by Davisson & Germer.
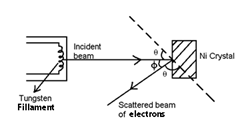
A beam of electron emitted by the electron gun is made to fall on nickel crystal. The energy of the incident beam of electron can be varied by charging the applied voltage to the electron gun.
The intensity of scattered beam of electron is measured as a function of the angle of scattering (φ).
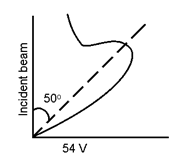
They found that when the incident beam of electrons accelerated through a potential of 54 V was made to incident on the nickel crystal; the intensity of scattered beam was maximum at an angle of 50°.
So θ + φ + θ = 180°
2θ = 180 – 50
θ = 65°
According to Bragg’s equation
n λ = 2 d sinθ (n=1 For first principal maxima)
λ = 2 x 0.91 sin 65° (For Ni d=0.91 A°)
λ = 1.65 A°
According to de-Broglie
λ = 12.27 A° / (V)1/2 = 12.27/(54)1/2 = 1.67 A°
Since two results are in close agreement whit each other so the experiments proves the wave nature of moving particles.
 SureDen
SureDen