Rutherfords Experiment
A Rutherford’s experiment on the scattering of ∝ particles (Geiger mariden Experiment):
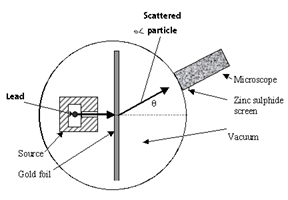
A radioactive source of ∝ - particle like Bismuth Bi is enclosed in thick block, provided with narrow the ∝-particle from this source are collimated into a narrow beam through a slit. The beam is allowed to fall on a thin gold foil of thickness 2.1 x 10-7 m. The alpha particle scattered in different direction which consists of a zinc sulphate screen and a microscope.
Observations:
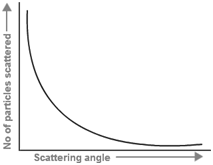
A graph is drawn between the scattering angle and the number N(θ) of the ∝-particle scattered at an angle θ very large number of ∝-particles.
The graph in fig.(b) reveals the following fact:
- Most of the ∝-particle pass straight through the gold foil and suffers in small deflections.
- A few ∝- particles about 1 in 8000 get deflected through 90° or more.
- Occasionally an ∝ particle gets rebounded from the gold foil suffering a deflection of nearly 180°.
Significance of result:
Rutherford concluded the following important facts about an atom:
- As most of ∝- particles pass straight through the foil so most of the space within the atom must be empty.
- To explain large angle scattering of alpha particles Rutherford suggested that all the positive charge and the mass of the atom is concentrated in a very small region called Nucleus of the atom.
3.The nucleus surrounded by a cloud of electrons whose total negative charge is equal to the total +ve charge on the nucleus so that the atom as whole is electrically neutral.
 SureDen
SureDen