Postulates of Bohrs Theory of Hydrogen
Postulates of Bohr’s theory of hydrogen atom:
This model also called planetary model of the atom is based on the following postulates:
(i) Nuclear concept: An atom consist of a small massive central core called nucleus around which planetary electrons revolves
The centripetal force required for their rotation is provided by electron static attraction between e- and the nucleus.
(ii) Quantum condition: of all the possible circular orbits allowed by the classical theory, the electrons are permitted to circulate only in those orbits in the angular momentum of an e- is on which integral multiple of (h/2π) whose h is u plank’s constant. Therefore, for any permitted orbit.
L = mvr = nh/2π, n = 1, 2, 3…………
Where n = Principle quantum number
(iii) Stationary orbits: While revolving in the (acceptable) Permissible orbits an electron does not radiate energy. These non radiating orbits are called stationary orbits.
(iv) Frequency Condition: An atom can emit the form of discrete energy photons only when an electron gimps from a higher to the lower orbit or from lower to higher orbit respectively if E1 and E2 are the en energies associated with these permitted orbit then the frequency (ν) of the emitted or absorbed radiation is given by
hν = E2 – E1
Bohr’s theory of hydrogen atom: (Radii of permitted (allowed) orbits in the hydrogen atom)
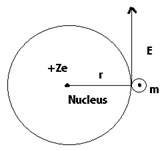
According to Bohr Theory of atom, H atom consist of nucleus with positive charge Z e and a single e- of charge –e which revolves around the nuclear in a circular orbit of radius r.
Here Z is the atomic number and for hydrogen atom Z = 1, The electrostatic force of attraction between the nucleus and the e- is
F = K q1q2/r2
F = K Ze.e/r2
F = K. Ze2/r2 —(1)
To keep the e- in its orbit, the centripetal force on the e- must be equal to the electrostatic, attraction therefore,
mV2/r = K. Ze2/r2 —(2)
r = K. Ze2/mv2 —(3)
Where m = mass of the electron and V is its speed in an orbit of radius r. Bohr’s quantization condition for angular momentum is mvr = nh/2π
r = nh/2πmv —(4)
From equation (3) and (4)
K. Ze2/mv2 = nh/2πmv
V = K 2πZe2/nh
Substituting this value of v in equation (4), we have
r = (nh/2πm) (nh/K 2πZe2)
r = n2h2/4π2 kmZe2 —(6)
as r is proportional to n2 the radii of permitted orbits increase in the ratio of 1 : 4 : 9 : 16………
The radius of the innermost orbit of the hydrogen atom called Bohr’s radius and can be determined by putting Z = 1 and n = 1 in equation (6) and it is denoted by ro. So equation (6) becomes
ro = n2/4π2m Ke2
Substituting the value of all the constants
ro = (6.63 x 10-34)2/4 x (3.14)2 x (9.1 x 10-31) x 9 x 109 x (1.6 x 10-19)
ro = 5.29 x 10-11 m
= 0.53 A°
 SureDen
SureDen