Voids
Coordination number: The number of adjacent particles of atoms is called coordination number.
In both ccp and hcp, each sphere is surrounded by 12 adjacent atoms, thus coordination number is equal to 12 in each case.
Formation of voids in close packing:
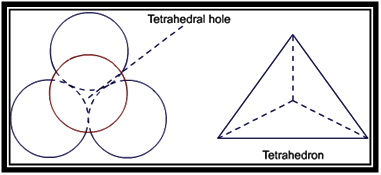
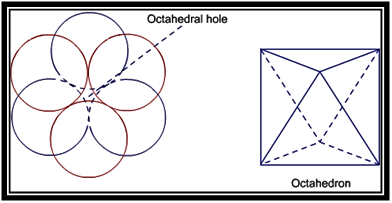
Empty space left after the packing is called void. Two types of voids are formed in ccp and hcp structures. These are tetrahedral voids and octahedral voids.
Tetrahedral voids are formed because of formation of tetrahedron between the layers of atoms. Thus, voids in the shape of tetrahedron are called tetrahedral voids.
Octahedral voids are formed because of formation of octahedron between the layers of atoms. Thus, voids in the shape of octahedron are called octahedral voids.
Number of voids:
The number of formation of voids depends upon the number of close packed spheres. The number of tetrahedral voids is formed twice as the number of octahedral voids while close packing of atoms in ccp and hcp structures.
Thus, if number of close packed spheres is equal to ‘N’.
Therefore, number of octahedral voids formed = N
And, the number of tetrahedral voids formed = 2N
Formula of a compound and number of voids filled:
Bigger ions, usually anions, form close packed structure and smaller ions, usually cations occupy the voids in ionic solids. If cations are bigger in size, they occupy octahedral voids and if are smaller enough then they occupy tetrahedral voids.
The occupation of number of voids depends upon the chemical formula of compound. It may be possible to occupy all the voids or fraction of voids.
 SureDen
SureDen