Oxides of Nitrogen
(6) Oxides:- The elements of group 15 combine directly or indirectly to form a large number of oxides. All the oxides of nitrogen (except NO and N2O) and phosphorous are strongly acidic. Oxides of Arsenic are weakly acidic oxides of Antimony are amphoteric and those of bismuth are weakly basic. It is because the size of nitrogen atom is small and it has a strong negative field hence the electron pair between O-H bond is strongly pulled to release H+. As the size increases this tendency goes on decreasing.
Structure of oxides of group 15 (oxides of Nitrogen)
(Nitrous Oxide/Laughing Gas)
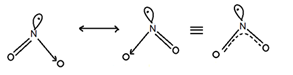
(Nitric Oxide)
Thus NO has odd electron (11 electrons) bond and is paramagnetic in gaseous state. But in solid or liquid (Low.Temp.) It is diamagnetic as it exists as a loose dimes in which all the electrons are paired.
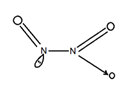
(iii) N2O3
(Dinitrogen Trioxide)
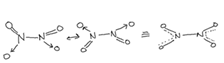
(Nitrogendioxide)
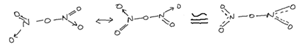
(Dinitrogen tetraoxide)
(Dinitrogen pentaoxide)
 SureDen
SureDen