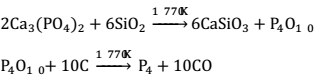Group 15 Element Phosphorus
PHOSPHORUS
The mineral family of phosphorus is called Apatites with general formula [Ca3(PO4)2]3. CaX2; X = F, Cl etc.
Phosphorus is obtained by heating the mineral with silica and coke in an electrical furnace at 1770 K.
The vapours of phosphorus thus obtained upon condensation give white phosphorus which exists as discrete P4 molecules.
Allotropes of Phosphorus:-
Phosphorus exists in many allotropic forms. Out of these white, red and black are important.
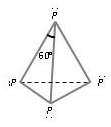
White Phosphorus:- White Phosphorus obtained as above exists as P4. The four phosphorus atoms lie at the corners of a regular tetrahedron with bond angle 60°. On exposure to light white phosphorus turns yellow and hence is also called yellow phosphorus. Because of angular strain in P4 molecule, it is highly reactive. It glows in dark; this property is known as Chemiluminescence or Phosphorescence.
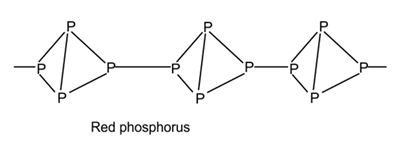
(2) Red Phosphorus:- Red Phosphorus is obtained by heating white phosphorus at 573K temperature in an inert atmosphere in absence of air. It exists as P4tetrahedral joined together by covalent bond to give polymeric structure.
It is less reactive than white phosphorus, as strain is relaxed and is non poisonous. It does not glow in air.
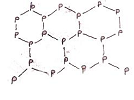
(3) Black Phosphorus:- Black Phosphorus has two forms α-black phosphorus and β-block phosphorus. α-black phosphorus is obtained when red phosphorus is heated in a sealed tube at 803K. It can be sublimed in air and has opaque monoclinic or rhombohedra crystals. It does not oxidize in air. β-phosphorus is prepared by heating white phosphorus at 473K under high pressure (4000 – 12000 atm.). It does not burn in air up to 673 K.
 SureDen
SureDen