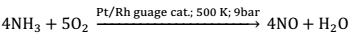Compounds of Group 15 Nitric Acid
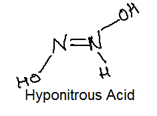
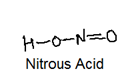
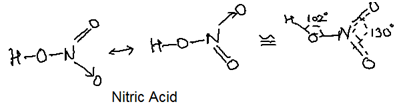
Nitrogen forms the oxo acids such as H2N2O2 (hyponitrous acid), HNO2 (Nitrous acid) and HNO3 (Nitric Acid) with their structures:
Preparation of Nitric acid:-
(1) Laboratory Preparation:- In laboratory, nitric acid is prepared by heating KNO3 or NaNO3 and conc H2SO4 in a glass retort.
KNO3 + H2SO4 → KHSO4 + HNO3
(2) Commercial Preparation:- On commercial scale, HNO3 is prepared by Ostwald’s process. This method is based upon the catalytic oxidation of ammonia by atmospheric oxygen.
Nitric oxide thus formed combines with oxygen to give NO2.
2NO + O2 → 2NO2
Nitrogen di – oxide so formed, dissolves in water to give HNO3.
3NO2 + H2O → 2HNO3 + NO
The NO gas is then recycled. The aq. HNO3 cannot be concentrated by distillation above 68% by mass due to formation of azeotropic mixture. Further conc. upto 98% can be achieved by dehydration with conc. H2SO4.
Physical Properties of Nitric Acid:-
Pure Nitric acid is colourless, fuming liquid with pungent smell and contains some dissolved oxides of nitrogen, hence is called Fuming Nitric Acid.
(2) It freezes at 231.4K and boils at 355.6K.
(3) Laboratory grade Nitric acid is a constant boiling mixture (394K) i.e. Azeotropic mixture containing 68% HNO3 and 32% H2O by mass. It has a specific gravity 1.504.
Chemical Properties:-
(1) Acidic nature:- Nitric Acid is a strong mono basic acid. In aqueous solution, it ionizes as:
HNO3 + H2O → H3o+ + NO3-
Hence it reacts with bases and oxide of metals to give nitrates and turns Blue litmus Red e.g.
CaO + 2HNO3 → Ca(NO3)2 + H2O
Na2CO3 + 2HNO3 → 2NaNO3 + CO2 + H2O
(2) Oxidation nature:- Nitric acid is a strong oxidizing agent and oxidizes most of the metals (except Gold & Pt.) and non metals. The products of oxidation depend upon the conc. of acid, temperature and nature of material undergoing oxidation. The process of oxidation can be explained as:
(a) Metals which are more electro positive than hydrogen (i.e. have –ve standard electrode Potential e.g. Na, K, Mg, Zn, Fe etc.), liberate nascent hydrogen which reduces nitric acid. Let us take the example of Zn
(i) Zn with dilute HNO3
Zn + 2HNO3 → Zn(NO3)2 + 2H] * 4
HNO3 + 8H → NH3 + 3H2O
NH3 + HNO3 → NH4NO
______________________________________
4Zn + 10HNO3 → 4Zn(NO3)2 + 3H2O + NH4NO3
______________________________________
However, on heating NH4NO3 decomposes as
NH4NO3 → N2O + 2H2O
Hence the overall reaction with Hot Dilute HNO3 becomes:-
4Zn + 10HNO3 → 4Zn(NO3)2 + 5H2O + N2O
(ii) Zn with conc. HNO3:
Zn + 2HNO3 → Zn(NO3)2 + 2H
HNO3 + H → H2O + NO2]*2
________________________________________
Zn + 4HNO3 → Zn(NO3)2 + 2H2O +2NO2
________________________________________
(b) Metals which are less electropositive than hydrogen (i.e. have +ve standard reduction potential e.g. Cu, Hg, Ag, etc.) do not liberate hydrogen, instead nitric acid librates nascent oxygen. e.g. Cu reacts with HNO3 as:
(i) Cu with dilute HNO3:
2HNO3 → H2O + 2NO + 3[O]
Cu + [O] → CuO]*3
CuO + 2HNO3 → Cu(NO3)2 + H2O]*3
_________________________________
3Cu + 8HNO3 → 3Cu(NO3)2 + 4H2O + 2NO
____________________________________
(ii) Cu with conc. HNO3:
2HNO3 → H2O + 2NO2 + [O]
Cu + [O] → CuO
CuO + 2HNO3 → Cu(NO3)2 + H2O
___________________________________
Cu + 4HNO3 → Cu(NO3)2 + 2H2O + 2NO2
(c) The Metals only magnesium and manganese react with very dilute HNO3 to give H2 gas. The metals iron chromium, Nickel and Aluminum when dipped in conc. HNO3 there develops a thin protective layer of the metal oxide on their surfaces. Hence they lose their normal activity and become passive. This phenomenon is known as Passivity.
(d) Non-Metals are not attacked by dilute nitric acid. But conc. Nitric and oxidizes many non-metals such as carbon, sulphur, iodine, phosphorous to their respective
oxo- acids, while nitric acid itself is reduced to NO2. e.g.
(i) Carbon is oxidized to carbonic acid (H2CO3):-
2HNO3 → H2O + 2NO2 + [O]*2
C + 2[O] + H2O → H2CO3
___________________________
C + 4HNO3 → H2CO3 + H2O + 4NO2
____________________________
(ii) Sulphur is oxidized to sulphuric acid (H2SO4):-
2HNO3 → H2O + 2NO2 + [O]*24
S8 + 24[O] + 8H2O → 8H2SO4
__________________________________
S8 + 48HNO3 → 8H2SO4 + 16H2O + 48NO2
__________________________________
(iii) Iodine is oxidized to Iodic acid (HIO3):-
2HNO3 → H2O + 2NO2 + [O]*5
I2 + 5[O] + H2O → 2HIO3
_________________________________
I2 + 10HNO3 → 2HIO3 + 4H2O + 10NO2
_________________________________
(iv) Phosphorus is oxidized to Phosphoric acid (H3PO4):-
2HNO3 → H2O + 2NO2 + [O]…… ] * 10
P4 + 10[O] + 6H2O → 4H3PO4
________________________________
P4 + 20HNO3 → 4H3PO4 + 4H2O + 2NO2
________________________________
Ring Test for Nitrate ion:- In qualitative analysis, the presence of nitrate ion is detected by ring test. In this test a freshly prepared solution of ferrous sulphate is added to the aqueous solution of a nitrate (salt). Pure conc. H2SO4 is then added carefully drop wise along the walls of test tube. The appearance of dark brown ring at the junction of the two layers indicates the presence of a nitrate ion.
NO-3 + 3Fe+2 + 4H+ → NO + 3Fe+3 + 2H2O
Fe+3 + NO + 5H2O → [Fe(H2O)5 NO+]+2
Penta-aqua-nitrosonium iron (I) ion (Brown Complex)
Uses of Nitric acid:- Nitric acid is used in
(i) Preparation of ammonium nitrate and basic calcium nitrate [CaO.Ca(NO3)2] which are used as fertilizers.
(ii) Manufacture of explosives and Pyrotechnics such as gun cotton, nitroglycerine, TNT, Picric acid etc.
(iii) Pickeling (cleaning) of stainless steel and etching of metals.
(iv) Rocket fuels as an oxidizer.
 SureDen
SureDen