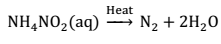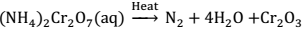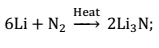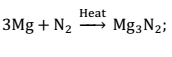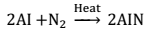Compounds of Group 15 DiNitrogen
COMPOUNDS OF GROUP – 15
DINITROGEN (N2)
Nitrogen was discovered by Daniel Rutherford. It exists as a diatomic gas (N2) in the elemental state, therefore, it is also called dinitrogen.
Methods of Preparation:-
(a) Commercial Preparation:- Dinitrogen is prepared commercially from air by liquefaction and fractional distillation. When liquid air is allowed to distil dinitrogen having lower B. Pt. (77.2K) distills first leaving behind liquid dioxygen (B.Pt. 90K).
(b) Laboratory Preparation:- Dinitrogen in the laboratory can be prepared by the following three methods:-
(i) By thermal decomposition of Ammonium nitrite:- When an equimolar aqueous solution of ammonium chloride and sodium nitrite is heated, ammonium nitrite is formed. It being unstable decomposes to form dinitrogen gas.
NH4Cl(aq) + NaNO2(aq) ⟶ NH4NO2(aq) + NaCl(aq)
Small amounts of NO and HNO3 are also formed, which can be removed by passing the gas through aqueous solution of sulphuric acid and Pot. dichromate.
(ii) From Ammonium dichromate:- Dinitrogen can also be prepared by thermal decomposition of ammonium dichromate.→
(iii) By thermal decomposition of sodium or barium azide:- Vary pure dinitrogen is obtained by this method.
Properties of Dinitrogen:-
(A) Physical Properties:-
(i) Dinitrogen is a colorless, odorless, tasteless non toxic gas. But animals die in it due to want of O2.
(ii) It has two stable isotopes: 14N and 15N.
(iii) It has low freezing (63.2 K) and boiling (77.2 K) point and is very slightly soluble in water.
(iv) It is adsorbed by activated charcoal.
(B) Chemical Properties:- Dinitrogen is highly unreactive (inert) at room temp. due to high bond dissociation energy (N ≡ N). However, reactivity increases at high temp. Some chemical Properties are discussed as below:-
(i) Action of Litmus:- It is neutral towards litmus.
(ii) Action of active metals:- Active metals when burnt in an atmosphere of nitrogen gas, they form their ionic nitrides.
(iii) Action of non-metals:- Dinitrogen reacts readily with hydrogen and oxygen gases.
(a) With hydrogen gas:- When a mixture of dinitrogen and dihydrogen is heated to about 700K and at a pressure of 200atm. In presence of iron as catalyst and Molybdenum as promoter ammonia is formed:-
N2(g) + 3H2(g) 2NH3(g)
This reaction forms the basis of Haber’s Process for the manufacture of ammonia.
(b) With oxygen gas:- Dinitrogen and dioxygen combine to form nitric oxide when the mixture is heated to 2273 – 3273 in an electric arc.
N2(g) + O2(g) 2NO
This reaction forms the basis of manufacture of Nitric Acid by Birkland & Eyde Process.
Uses of Dinitrogen:-
(i) It is used in the manufacture of nitric acid, ammonia etc.
(ii) It is used to prepare calcium cyanamide which is used as fertilizer under the name nitrolim (CaCN2 + C)
(iii) Dinitrogen is used to create inert atmosphere.
(iv) Liquid dinitrogen is used to preserve biological materials, food materials and in cryosurgery.
(v) It is used in gas filled thermometers to measure high temp.
 SureDen
SureDen