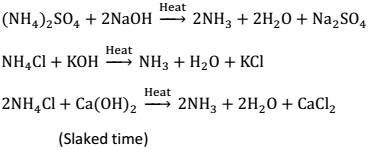Compounds of Group 15 Ammonia
Ammonia is present in small quantities in air and soil where it is formed by the decay of nitrogenous organic matter such as urea.
NH2CONH2 + 2H2O → (NH4)2CO3 → 2NH3 + H2O + CO2
Methods of preparation:-
(1) By heating ammonium salts with bases:
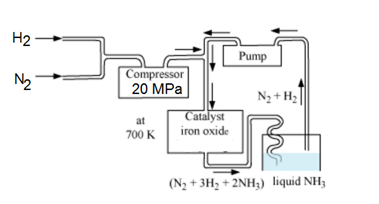
(2) By Haber’s Process (Commercial preparation):-
On a commercial scale, ammonia is prepared by mixing N2 and H2 under a suitable condition of temp. and pressure.
N2(g) + 3H2(g) 2NH3(g)
ΔH= -92.4 KJ/mole
It is an equilibrium reaction and the favorable conditions are determined by Le Chatelier’s Principle:
(a) Low Temperature:- Since the forward reaction is exothermic low temperature will favour the formation of ammonia. However, at low temperature, the rate of reaction is slow. The optimum temperature for the reaction has been found to be around 700 K.
(b) High Pressure:- Since the forward reaction occurs will decrease in volume, high pressure will favour the formation of ammonia. The reaction is usually carried out at a pressure of about 200 atm. (200 * 105 Pa).
(c) Catalyst:- The rate of reaction is fairly low around 700 K. Its rate is increase by using iron oxide as catalyst with small amount of K2O and Al2O3. Sometimes, molybdenum is used as promoter
Properties of Ammonia:- In ammonia Nitrogen atom is sp3 hybridized. It has one Ione pair of electrons and three bond pairs of electrons. Since the Ione pair – bond pair repulsion is stronger than bond pair – bond pair repulsion, therefore H – N – H bond angle decreases from 109°28’ to 107.8°. Thus NH3 molecule has a pyramidal geometry with N – H bond length of 101.7 pm
Ammonia is a colourless gas with pungent (Ammonical) smell and causes tears in the eyes. It is highly soluble in water (1000 parts in 1 part of water).
Chemical Properties:-
(1) Basic nature:- Ammonia is highly soluble in water. Its aqueous solution is weakly basic due to formation of OH- ions.
NH3(g) + H2O(I) NH+4(aq) + OH-(aq)
It turns red litmus blue and neutralizes acids.
NH3 + HCl → NH4Cl
2NH4OH + H2SO4 → (NH4)2SO4 + 2H2O
(2) Reaction with heavy metals salt solution:- Ammonium hydroxide reacts with many metallic salts and precipitates than as hydroxides:
FeCl3(aq) + 3NH4OH(aq) → Fe(OH)3(S)↓ + 3NH4Cl(aq)
(Brown Ppt.)
AlCl3(aq) + 3NH4OH(aq) → Al(OH)3(S)↓ + 3NH4Cl(aq)
(White Ppt.)
CrCl3(aq) + 3NH4OH(aq) → Cr(OH)3(S)↓ + 3NH4Cl(aq)
(Green Ppt.)
This property is used in precipitating these metals as their hydroxides in the group III of qualitative analysis.
(3) As a Lewis base:- The presence of Ione pair of electrons on the nitrogen atom of ammonia molecule makes it a Lewis base. It donates the electron pair and forms linkages with metal ions and the formation of such complex compounds finds application in the detection of metal ions such as Cu+2, Ag+:
Cu+2(aq) + 4NH3(aq) → [Cu(NH3)4]+2(aq)
Blue Deep blue
Ag+(aq) + Cl-(aq) → AgCl(S)
Colourless White Ppt.
AgCl+ 2NH3 → [Ag(NH3)2]Cl(aq)
White Ppt. Colourless
Uses of Ammonia:- Ammonia is used as:
(i) In the formation of various nitrogenous fertilizers such as urea, ammonium nitrate, ammonium sulphate, calcium ammonium nitrate (CAN) etc.
(ii) In the manufacture of Inorganic compounds such as HNO3, Na2CO3 etc.
(iii) Liquid ammonia is used as refrigerant in ice – factories and cold storages.
 SureDen
SureDen