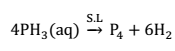Compound of Phosphorus
PHOSPHONE (PH3)
Phosphine is prepared by the reaction of calcium phosphide with water or dilute HCl.
Ca3P2 + 6H2O → 3Ca(OH)2 + 2PH3
Ca3P2 + 6HCl → 3CaCl2 + 2PH3
In Laboratory, it is prepared by heating white phosphorous with conc. NaOH solution in an inert atmosphere of CO2.
P4 + 3NaOH + 3H2O → PH3 + 3NaH2PO2
(Sodium hypophosphide)
Pure PH3 is not inflammable, but catches fire in air due to the presence of P2H4 (diphosphine) or P4 vapours. To purify it form impurities, it is absorbed in HI to form phosphonium iodine (PH4I) which on treating with KOH gives off Phosphine. PH3 + HI → PH4I
PH4I + KOH → KI + H2O + PH3
Properties:-
(1) Physical Nature:- It is a colourless, unpleasant smelling (garlic or rotten fish), and highly poisonous gas.
(2) Solubility:- Phosphine gas is slightly soluble in water. The solution of phosphine in water decomposes in presence of air and light to give phosphorous and H2.
(3) Basic Nature:- Phosphine is weakly basic in nature and gives phosphonium compounds with acids:
PH3 + HBr → PH4Br
(4) Reaction with CuSO4 & Mercuric Chloride (HgCl2):- When absorbed in these salts corresponding phosphides are formed:
3CuSO4 + 2PH3 → Cu3P2 + 3H2SO4
3HgCl2 + 2PH3 → Hg3P2 + 6HCl
Uses:-
(1) It is used as Holmes’s signals in deep seas and oceans for signaling danger points to steamers. Container containing a mixture of calcium carbide and calcium phosphide are pierced and put near danger point. In contact with water, a mixture of phosphine and acetylene is formed. Phosphine contains the traces of di-phosphine (P2H4) which is highly inflammable and catches fire. This ignites acetylene which burns with a luminous flame and thus serves as a signal to the approaching ship.
(2) For the production of smoke screens: Shells containing calcium phosphide are exsploded by war – ships, which burn in air to give clouds of P4O10 which acts as smoke screen.
PHOSPHORUS HALIDES
Phosphorus forms two halides PX3 (trihalides) and PX5 (penta halides).
Phosphorus Trichloride (PCl3):-
Phosphorus Trichloride is prepared by the action of thionyl chloride on white phosphorous:
P4 + 8SOCl2 → 4PCl3 + 4SO2 + 2S2Cl2
In laboratory, it is prepared by heating white phosphorus in a current of dry chlorine when it is distilled.
P4 + 3Cl2 → 4PCl3
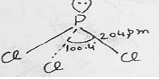
Structure:- In PCl8, P atom is sp3 hybridized, having a lone pair of electrons over it. The bond angle is 100.5° due to larger size of chlorine atom.
Properties:- It is colourless pungent smelling liquid with following chemical properties.
(1) Action with water:- It reacts violently with water to produce phosphorous acid and hydrochloric acid.
PCl3 + 3H2O → H3PO3 + 3HCl
Due to the formation of HCl, it fumes with water:-
(2) Reaction with hydroxyl and carboxylic group:- PCl3 replaces these groups with chlorine e.g.
3CH3CH2OH + PCl3 → 3CH3CH2Cl + H3PO3
3CH3COOH + PCl3 → 3CH3COCl + H3PO3
Phosphorus Penta Chloride PCl5:-
Phosphorous penta chloride is prepared by reaction of Cl2 with P4 and PCl3.
P4 + 10Cl2 → 4PCl5
PCl3 + Cl2 → PCl5
It is also prepared by the action of sulphuryl chloride (SO2Cl2) on white phosphorous.
P4 + 10SO2Cl2 → 4PCl5 + 10SO2
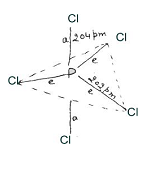
Structure:- In PCl5 phosphorous undergoes sp3 d-hybridization and has trigonalbipyramidal geometry in gaseous and liquid states. It has three equatorial bonds and two axial bonds. Since three equatorial bonds are repelled by two axial bonds; and two axial bonds are repelled by three equatorial bonds. Hence axial bond lengths suffer more repulsion and are greater than equatorial bond lengths.
Properties:- Phosphorus pentachloride is a pale yellow crystalline solid with a characteristic pungent smell.
In the solid state it exists as an ionic solid, [PCl4]+[PCl6]- in which the cation [PCl4]+ is tetrahedral and the anion [PCl6]- is octahedral. The major chemical properties are:
(i) Dissociation:- On heating PCl5 dissociates into PCl3 and Cl2
PCl5 ⇌ PCl3 + Cl2
(ii) Action of water (Moisture):-
PCl5 + H2O → POCl3 + 2HCl
PCl5 + 4H2O → H3PO4 + 5HCl
(Excess)
Due to the formation of HCl it fumes in water (Moist air).
(iii) Reaction with metals:- Metals on heating with PCl5 form corresponding chlorides.
Ag + PCl5 → 2AgCl + PCl3
Sn + 2PCl5 → SnCl4 + 2PCl3
(iv) Reaction with compounds containing hydroxyl groups:- The compound is replaced by Cl – group
CH3COOH + PCl5 → CH3COCl + POCl3 + HCl
CH3CH2OH + PCl5 → CH3CH2Cl + POCl3 + HCl
Uses:- PCl5 as well as PCl3 is used as an important reagent in organic chemistry.
 SureDen
SureDen