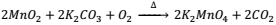Potassium Permanganate
Potassium Permanganate (KMnO4)
Preparation:- Commercially Pot. Permanganate is prepared from mineral pyrolusite (MnO2) in the following steps:
a)Conversion of MnO2 into potassium manganate
b) Oxidation of K2MnO4 into permanganate
2K2MnO4 + Cl2 ⟶ 2KMnO4 + 2KCl
Properties:-
- Colour:- It is a deep purple black crystalline solid with M. Pt. 523K.
- Solubility:- It is moderately soluble in water at room temp. and more soluble in Hot Water.
-
Action of Heat:- It forms different products at different conditions e.g.
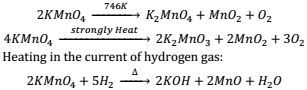
- Action of Conc. H2SO4:-
- In cold:-
2KMnO4 + 2H2SO4 ⟶ Mn2O7 + 2KHSO4 + H2O
- On warming:-
4KMnO4 + 6H2SO4 ⟶ 2K2SO4 + 4MnSO4 + 6H2O + 5O2
5. Oxidising Properties:- KMnO4 acts as strong oxidizing agent in neutral, basic as well as acidic medium.
- In neutral medium 2KMnO4 + H2O ⟶ 2KOH + 2MnO2 + 3[O]
Eq. wt. in neutral medium:- (2 x (KMnO4) / 3[O] x 8 = (2/3) (158/16) x 8 = 52.67
- In basic medium:-
2KMnO4 + 2KOH ⟶ 2K2MnO4 + H2O + O
K2MnO4 + H2O ⟶ MnO2 + 2KOH + O ] x 2
________________________________
2KMnO4 + H2O ⟶ 2KOH + 2MnO2 + 3O
The over all equation is similar to aqueous medium. Hence eq. wt. in basic medium is also 52.67. If the strong Alkali is used only the first feaction is occurs and the eq. wt. is 158.
iii. In acidic Medium:-
2KMnO4 + 3H2SO4 ⟶ K2SO4 + 2MnSO4 + 3H2O + 5 [O]
Eq. wt. in acidic medium = 2 x (KMnO4) / 5[O] x 8 = (2 x 158)/(5 x 16) x 8 = 31.6
Note:- Eq. wt. is the wt. of substance which combines or displaced directly or indirectly 8 parts by wt. of oxygen, 35.5 parts by wt of chlorine or 1 part by wt. of hydrogen.
6. Examples of Oxidation:-
-
Oxidation of H2S into S:-
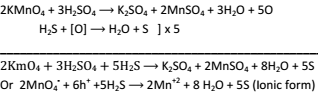
-
Sulphur dioxide into H2SO4:-
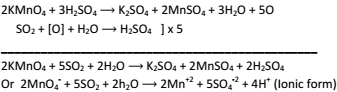
-
Ferrous Sulphate into Ferric sulphate:-
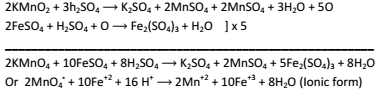
-
H2O2 to H2O and C
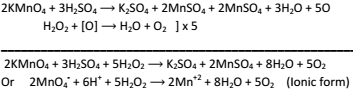
-
Potassium Iodide to Iodine:-
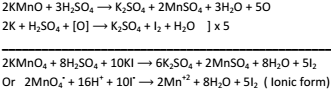
-
Uses: -
- It is used as reagent in volumetric analysis.
- It is used as an oxidizing agent in Laboratory.
- It is used as a disinfectant and germicide.
- It is used in purification of water.
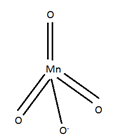
Structure: Mn in MnO4- is sp3 hybridized. The four oxygens are tetrahedral attached to Mn.
 SureDen
SureDen