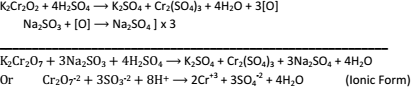Potassium Dichromate
Potassium Dichromate (K2Cr2O7)
Preparation:- Potassium dichromate is prepared from the ore called chromite ore or ferrochrome or chrome ion (FeO.Cr2O3) in following steps.
- Conversion of chrome iron into sodium chromate by heating with sodium carbonate
- Conversion of Sodium Chromate into sodium dichromate by the action of H2SO4 2Na2CrO2 + H2SO4(con.) → Na2Cr2O7 + Na2SO4 + H2O
- Conversion of Sod. Dichromate into potassium dichromate by the action of KCl
Na2Cr2O7 + 2KCl → K2Cr2O7 + 2NaCl
(pot. Dichromate)
Properties:-
- Colour and M.Pt:- It forms orange coloured crystals melting at 669K.
- Solubility:- It is moderately soluble in cold water but freely soluble in hot water.
-
Action of heat:- When heated K2Cr2O7 decomposes to give oxygen.
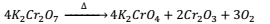
-
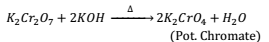
Action of Alkalis:- When alkali is added to orange red colored solution of Pot. Dichromate a yellow solution of Pot. Chromate is obtained and the reaction can be reversed by adding acid to it.
- Action of Concentrated Sulphuric Acid:-
(a) In Cold:- K2Cr2O7 + 2H2SO4(dil.) → 2KHSO4 + 2CrO3 + H2O
(b) In Hot:- 2K2Cr2O7 + 8H2SO4 →2K2SO4 + 2Cr2(SO4)3 + 8H2O + 3O2
- Oxidizing Properties:- K2Cr2O7 is very powerful oxidizing agent in acidic medium. For example:Oxidation of Kl liberate I2:
K2Cr2O7 + 4H2So4 → K2SO4 + Cr2(SO4)3 + 4H2O +3[O]
2Kl + H2SO4 + O → K2SO4 + I2 + H2O] x 3
------------------------------------------------------------------------
K2Cr2O7 + 6KI + 7H2SO4 → 4K2SO4 + Cr2(SO4)3 + 7H2O + 3I2
Or Cr2O7-2 + 6I- + 4H+ → 2Cr+3 + 7H2O + 3I2 (Ionic form).
- Oxidation of Ferrous salt to ferric salt:
K2Cr2O7 + 4H2SO → K2SO4 + Cr2(SO4)3 + 4H2O + 3[O]
2FeSO4 + H2SO4 + H2SO4 + O → Fe2(SO4)3 + H2O ] x 3
____________________________________________________________________
K2Cr2O7 + 7H2SO4 + 6FeSO4 → K2SO4 + Cr2(SO4)3 + 3 Fe2(SO4)3 + 7H2O
Or Cr2o7-2 + 6 Fe+2 + 14 H+ → 2Cr+3 + 6Fe+3 + 7H2O (Ionic form)
- Oxidation of H2S to sulphur:-
- Oxidation of Sulphites to sulphates:
- Oxidation of SO2 to sulphuric acid:
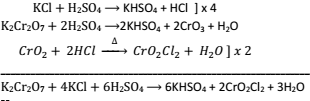
7.Chemistry of chromyl chloride test:- It is used as a confirmatory test for chloride radicals. The salt containing chlorine is shook with conc. H2SO4 and then heated – A radish brown vapours of chromyl chloride are obtained.
Uses:-
It is used in volumetric analysis.
It is used in chrome tanning in lather industry.
It is used in Calico printing and dyeing
It is used as an oxidizing agent in organic chemistry.
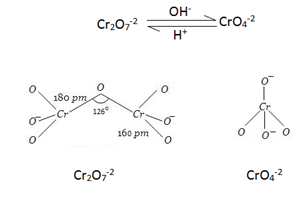
Structure:- Chromate ion (CrO4-2) and dichromate ion (Cr2O7-2) exist in equilibrium at a pH of 4.
They are interconvertible by change in pH.
 SureDen
SureDen