Introduction
d and f-block Elements
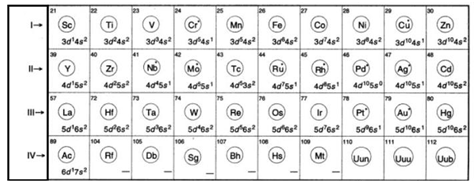
The elements in the periodic table present in between the s and p-block elements (Representative Elements) are transition elements. These elements are also called the d-block elements because the last electron in these elements enters into d-orbital.
Electronic Configuration:- The Transition elements are categorized into four transition series.
The general valence shell electronic configuration of transition elements is (n – 1) d1-10 ns0-2 (n-represents the valence shell). In some of the elements s-orbital has one or zero electron, it is due to its tendency to make the d-orbital exactly half filled or full filled.
Definition of transition elements:- A transition elements is defined as the element whose atom in ground state or ion in one of the common oxidation states has incomplete (partly filled d-sub shell i.e. has 1-9 electrons in outermost d-sub shell. Copper -29 (Cu=3d104s1) is considered as transition element as in its most common oxidation state (Cu+2). It has partly filled d-orbital (3d9 4s0). Zn, Cd and Hg are not considered as transition elements because they neither in their atomic form nor in their common oxidation state (+2) have partly filled d-orbitals. However they are studied with transition elements just to maintain a rational classification of elements.
 SureDen
SureDen