Types of nucleophilic substitution reactions
Types of nucleophilic substitution reactions
The nucleophilic substitution reactions can be classified into two types:
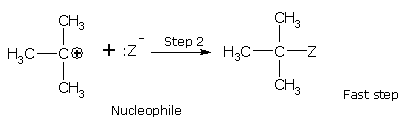
First is the SN1 reaction (substitution nucleophilic, first order). Reaction of an alcohol with thionyl chloride converts the OH group into chloride,This reaction proceds with retention of configuration and known as SN1, This type of reaction proceeds in two steps as:
MECHANISM
The first step is slow and is the rate-determining step. As the nucleophile (Z-) is not involved in the rate-determining step, the reaction depends only upon the concentration of alkyl halide (RX) and is, therefore, a first order reaction.
Rate = k [RX]
The order of reactivity depends upon the stability of carbonium ion formed in the first step. Since the 3° carbonium ion is most stable, the ionization of tertiary alkyl halide is favored. The order of reactivity for SN1 reaction is, tertiary > secondary > primary.
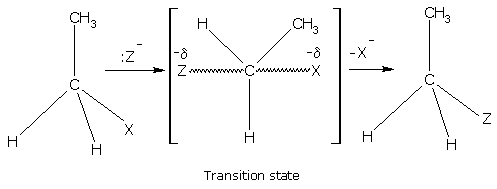
The second type is the SN2 reaction (substitution nucleophilic, second order). This type of reaction occurs in one step through the formation of transition state as: Rate =(SUBSTRATE)(NUCLEOPHILE)
IN SN2 MOSTLY INVERSION Take place,Here, the rate of reaction depends upon the concentration of both the alkyl halide and the nucleophile.
Rate = k[RX] [Z-]
The transition state from tertiary alkyl halide is less stable due to steric hindrance i.e., crowding of bulky groups. The order of reactivity is: primary > secondary > tertiary.
Thus the reactivity by SN1 versus SN2 increases as follows:
 SureDen
SureDen