Steriochemistry
Stereochemical aspects of nucleophilic substitution reaction:
(Optical Isomerism)
As SN2 produce with complete inversion while an SN1 produces Racemisation. So understand this concept we must know principles of stereochemistry.
- Plane polarized light
- Optical activity
- Enantiomersim
- Chirality
- Retention
- Inversion
- Racemisation
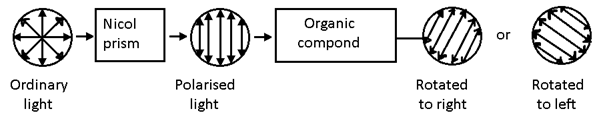
Plane polarized light:
- The light (monochromatic) whose vibrations occurs only in one plane is termed as plane polarized.
- Device that beings polarization in light in called a polarizer.
- Substances that have the ability to relate the plane polarized light either clockwise ( right ) or anti-clock (left ) are termed as optically active substance.
Apparatus to measure the extent of rotation of polarized light is called polarimeter.
An reaction proceeds with complete inversion of configuration while an
reaction proceeds with racemisation. To understand these concepts, we must know some basic principles of stereochemistry (Optical isomerism) and notations such as plane polarized light, optical activity, enantiomerism, chirality, retention, inversion and racemization.
- Nature of the substance,
- Wavelength of the light used (λ),
- Concentration of the solution (C) in g-cm-3,
- Nature of the solvent,
- Length of the path through which polarized light passes,
- Temperature at which the measurements are made.
Compounds having similar physical and chemical properties but differing only in the behavior towards polarized light are called optical isomers and this phenomenon is known as optical isomerism.
On basis of:-
- The optical isomer which rotates the plane polarized light to the right is known as dextrorotatory isomer. (d- form or indicated by +ve sign.)
- The optical isomer which rotates the plane polarized light to the left is known as Laevorotatory isomer. (l- form or indicated by –ve sign.)
Such (+) and (-) isomers of a compound are called Optical isomers and the phenomenon is termed as Optical isomerism.
- The optical powers of the above two isomers are equal in magnitude but opposite in sign. An equimolar mixture of the two forms, therefore, will be optically inactive. This mixture is termed as racemic mixture or dl- form or (±) mixture.
Lactic acid, tartaric acid, malic acid, amyl alcohol, glucose, sucrose are some of the organic compounds (chiral molecules) which show optical isomerism.
 SureDen
SureDen