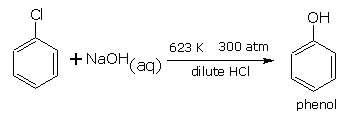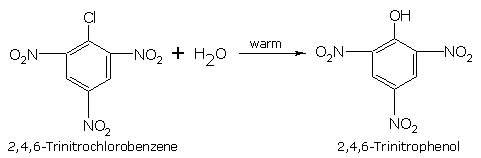Reaction with Na Mg and Chloral
REACTION with Na, Mg and chloral
Haloarenes are chemically less reactive than haloalkanes. They can undergo the following reactions:
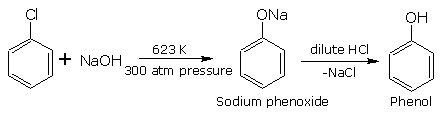
Replacement by hydroxy group (formation of phenol)
When aryl halides is heated under high temperature and under pressure (300 atm), with aq. solution of sodium hydroxide, the halogen atom is replaced by hydroxyl group forming phenol. Firstly sodium phenoxide is formed, which on acidification gives phenol.
THIS REACTION IS USED FOR THE MANUFACTURE OF PHENOL BY DOW”s PROCESS .
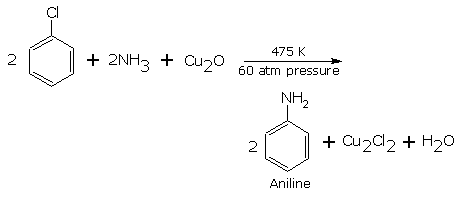
Substitution by amino group (formation of aniline)
The halogen atom is replaced by amino group when aryl halide IS heated with aqueous ammonia in the presence of a, catalyst cuprous oxide at 475 K And a pressure of 60 atm.,
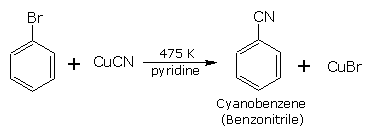
Substitution by cyano group (formation of cyanobenzene)
The halogen atom is replaced by cyano group on heating with anhy . cuprous cyanide in the presence of pyridine or dimethylformamide (DMF).
The cyanobenzene can be converted into Benzamide, benzoic aid and benzylamine under different conditions.
Effect of substituents in haloarenes (aryl halides) on the reactivity
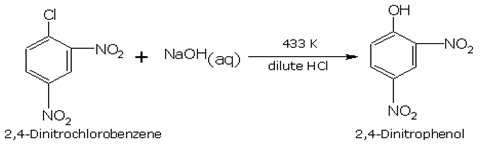
The reactivity of haloarenes is affected by the presence of certain groups at certain positions of the ring. The presence of electron withdrawing groups such as -NO2, -CN, -COOH, etc. at 'o' and 'p' positions to the halogen atom, greatly activates the halogen towards nucleophilic substitution reactions. For example, chlorobenzene is converted into phenol by aqueous NaOH only at temperatures above 573 K, whereas 'p' chloronitrobenzene is converted into nitrophenol by aqueous NaOH at a lower temperature of 433K. As the number of ortho and para nitro groups on the ring is increased the reactivity increases.
Nitro group which is meta to the chlorine has no effect on the reactivity but ortho and para have their effects .
Reaction of magnesium
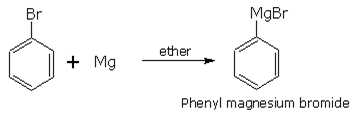
Aryl bromides and iodides react with magnesium in dry ether to form Grignard reagent . Aryl halide also form Grignard reagent in similar reaction,.
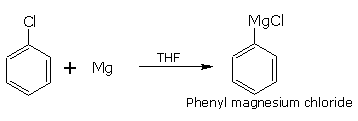
Chlorobenzene reacts with magnesium in the presence of tetrahydrofuran (THF) as solvent.
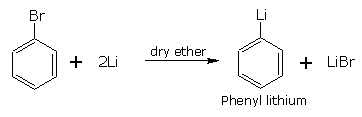
Reaction with lithium
Bromo and iodoarenes react with lithium metal in the presence of dry ether to form corresponding organo metallic compounds.(Formation of alkyl lithium )
Reaction with sodium :
When only haloarenes are treated wuth sodium in dry ether , it forms diphenyl ,This reaction is called Wittig Reaction.
However, when haloarenes react with sodium in the presence of ether, diphenyl is formed. This reaction is called 'Fittig reaction'.
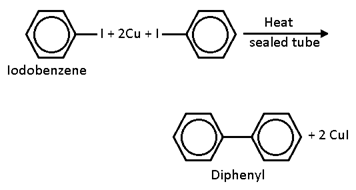
Reaction with copper powder
When Iodobenzene is treated with Copper Powder at 473 k in a sealed tube , diphenyl is formed.This reaction is called ULMAN REACTION.
 SureDen
SureDen