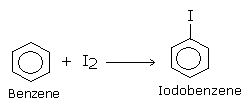Preparation of haloarenes
Preparation of haloarenes
Preparative methods of haloarenes are:
Direct halogenation of aromatic ring
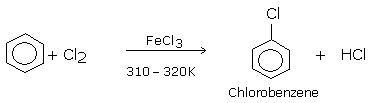
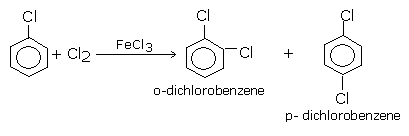
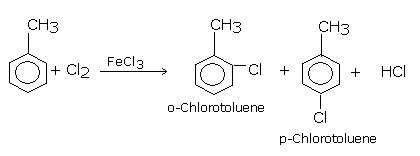
Direct chlorination or bromination of benzene or other aromatic hydrocarbon gives chloroarenes and bromoarenes. These reactions are carried out in the presence of Lewis acids such as ferric or aluminium halides (FeCl3, FeBr3, AlCl3) in the dark, at ordinary temperatures (310-320 K).Or in the presence of catalyst and halogen carrier.
The Lewis acid acts as a catalyst or a halogen carrier, as its function is to carry the halogen to the aromatic hydrocarbon.
MECHANISM OF HALOGENATION
Halogenation of Benzene is an electrophilic substitution reaction. The function of the halogen carrier is to generate 'electrophile' which attacks the benzene ring to form the product.
With excess halogen, the second halogen also gets introduced in the ring at ortho and para positions with respect to the first halogen because halogens are ortho and para directing groups.
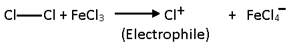
Step 1
The electrophile, i.e., halonium ion (Cl+, Br+ or I+) is generated by the action of Lewis acid (FeCl3 or anhyd. AlCl3….. etc.) on the halogens.
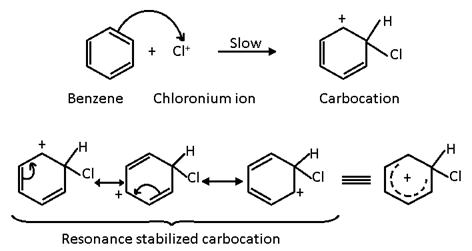
Step 2
The electrophile (Cl+) attacks the benzene ring to form an intermediate known as σ- complex or a carbocation (arenium ion) which is stabilized by resonance.
The formation of intermediate arenium ion (carbocation) is slow and hence is the rate determining step of the reaction.
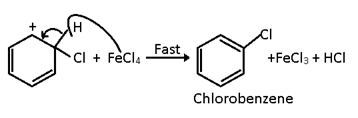
Step 3
The carbocation loses a proton (H+) to the base FeCl4- to give chlorobenzene.
The step is fast and hence does not affect the rate of the reaction.
Similarly, chlorination of toluene gives a mixture of ortho and para chlorotoluene because -CH3 group in toluene is ortho and para directing.
The reaction with fluorine is violent and cannot be controlled. Therefore, fluoroarene cannot be prepared by direct fluorination of aromatic hydrocarbon. Bromo compounds can be prepared in a similar way by reacting with Br2 in the presence of FeBr3. Iodoarenes are also difficult to prepare by direct iodination because the reaction is reversible and HI produced is a strong reducing agent to reduce iodobenzene back to benzene.
This reaction is carried out in the presence of an oxidizing agent like iodic acid or nitric acid, which oxidizes HI formed in the reaction to iodine enabling the reaction to proceed in the forward direction.
 SureDen
SureDen