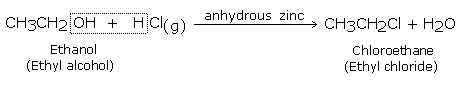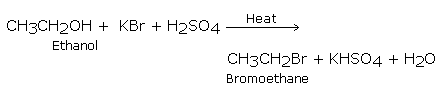Preparation of haloalkanes Part 2
Preparation of haloalkanes Part 2
From Alcohol:
Alcohol undergoes nucleophilic substitution reaction by halogen acid to give Haloalkanes.Tertiary alkanol reacts with hydrochloric acid directly to produce tertiary chloroalkane, but if primary or secondary alkanol is used, an activator such as zinc chloride is needed. This reaction is exploited in the Lucas test.
The most popular conversion is effected by reacting the alcohol with thionyl chloride (SOCl2) in the "Darzens halogenation," which is one of the most convenient laboratory methods because the byproducts are gaseous. Both phosphorus pentachloride (PCl5) and phosphorus trichloride (PCl3) also convert the hydroxyl group to the chloride.
Primary and secondary alcohols form chloroalkanes when hydrochloric acid gas is passed through alcohol in the presence of anhydrous zinc chloride (Groove's process).
The order of reactivity of halogen acids on alcohols is in accordance with the bond dissociation energies of H-X bonds: HI > HBr > HCl
Reactivity of alcohols towards this reaction is: tertiary > secondary > primary
The secondary and tertiary bromides and iodides unlike alkyl halides, cannot be prepared from the respective alcohols. This is because the secondary and tertiary alcohols undergo dehydration on heating with concentrated H2SO4 to form alkenes. The preparation of fluoroalkanes is not practical by this method.
Primary iodides and bromides can be obtained by heating an alcohol with hydrobromic acid (48%) and heating alcohols with constant boiling hydroiodic acid (57%) generated in situ by the action of phosphoric acid on potassium iodide.
From carboxylic acids
Two methods for the synthesis of haloalkanes from carboxylic acids are the Hunsdiecker reaction and the Kochi reaction.
Hunsdiecker reaction
The Hunsdiecker reaction (also called the Borodin reaction after Alexander Borodin) is the organic reaction of silver salts of carboxylic acids with halogens to give organic halides. It is an example of a halogenation reaction. The reaction is named after Heinz Hunsdiecker and Cläre Hunsdiecker
Kochi reaction
The Kochi reaction is an organic reaction for the decarboxylation of carboxylic acids to alkyl halides with lead tetraacetate and a lithium chloride or other lithium salts.
 SureDen
SureDen