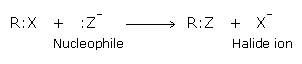Physical properties of haloarenes
Physical properties of haloarenes or aryl halides .
For the same halogen atom, the melting and boiling points increase as the size of the aryl group increase.
(i) Physical state: Haloarenes are colourless liquid or crystalline solid with characterstic smell
(ii) Solubility:Due to heavier in size , They are insoluble in water, but dissolve readily in organic solvents. Insolubility is due to inability to form hydrogen bonding in water. Para isomer is less soluble than ortho isomer.
(iii) Halo-arenes are heavier than water.
(iv) B.P. of halo-arenes follow the trend. Iodo arene > Bromo arene > Chloro arene. The boiling point of isomeric di haloarene are nearly equal .
Melting Point: their melting point varies because in symmetrical structure ,molecule are closely packed in crystal lattice .as a result , intermolecular forces of attraction are stronger and therefore , greater energy is required to break lattice . so it requires higher temperature to melt .
DIPOLE MOMENT : Fluoro benzene <chloro benzene <bromo benzene= iodo benzene
Low reactivity of haloarene as compound to haloalkane in term of nucleophilic substitution reaction
Nucleophilic substitution reactions
In the C-X bond there is a partial positive charge on the carbon atom and negative on the halogen atom. Thus nucleophilies attack the electron deficient carbon resulting in the displacement of the weaker nucleophile, the halide ion. Reactions of alkyl halides are generally nucleophilic substitution reactions.
The halide ions are substituted only if the attacking nucleophile is stronger. As the halide ion itself is a very weak nucleophile, the attacking nucleophile should be stronger than it. The order of reactivity of various alkyl halides towards nucleophilic substitution is:
RI > RBr > RCl > RF
This order of reactivity can be explained on the basis of strength of C-X bond. The C-X bond is the weakest in R-I and strongest in R-F.
These reactions take place either by the SN1 and SN2 mechanisms.
 SureDen
SureDen