Nucleophillic Subsitution of Haloarenes
Nucleophilic substitution reactions of haloarenes are given below, where halogen atom is replaced by other atoms or groups.
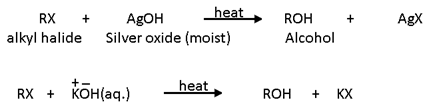
1. Replacement by hydroxyl (-OH) group (Formation of alcohols): Haloalkanes are hydrolysed to corresponding alcohols by moist silver oxide (AgOH) or by boiling with aqueous alkali solution (NaOH or KOH). The attacking nucleophile is OH
[ With the help of this reaction an alkene can be converted into alcohol. Alkene is first reacted with HBr to form alkyl bromide and then hydrolysis is done:
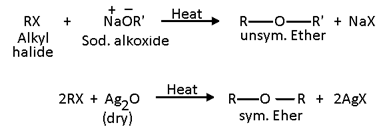
2. Replacement by alkoxy (-OR) group (Formation of ethers): Haloalkanes on heating with sodium or potassium alkoxides or with dry silver oxide (Ag2O) form ethers. This reaction is known as Williamson’s synthesis and the attacking nucleophile is OR-.
 SureDen
SureDen