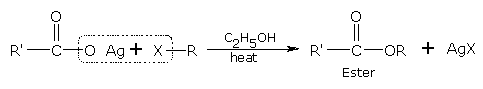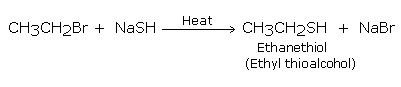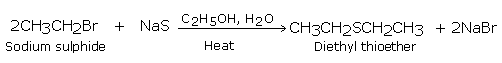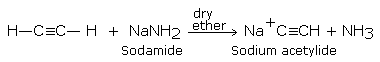Nucleophilic Reactions of Haloalkanes 2
Substitution by Nitrite Group
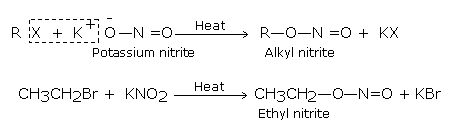
The halogen atom of haloalkane gets substituted by nitrite group (-O-N=O) when it is treated with sodium or potassium nitrite and forms alkyl nitrites.
The bond between K-O in KNO2 (alkyl nitrites) is ionic and therefore the negative charge on oxygen is the attacking site: it therefore forms nitrites (R-O-N = O).
Substitution by Nitro Group
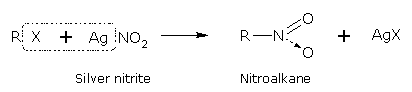
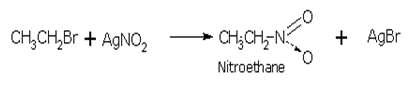
The halogen atom in haloalkane is replaced by nitro group (-NO2) when it is treated with silver nitrite (AgNO2) and give nitroalkanes.
The bond between Ag-O is covalent and therefore, the nucleophilic attack here occurs through the lone pair on nitrogen: nitroalkanes (R-NO2) are therefore, formed.
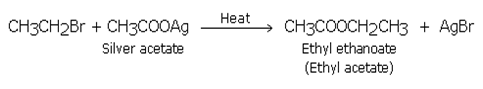
Substitution by carboxyl group (formation of esters)
When Haloalkanes are heated with an ethanolic solution of silver salt of a fatty acid these form esters.
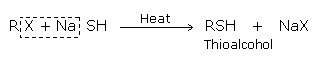
Substitution by hydrosulphide group
The formation of thioalcohols or thiols takes place when haloalkane is treated with-sodium or potassium hydrogen sulphide: the halogen atom gets replaced by hydrosulphide (-SH) group.
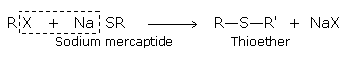
Substitution by Mercaptide Group
On treatment with mercaptide ion (RS-) haloalkanes give thioethers.
Thioethers are also formed by heating the haloalkanes with sodium or potassium sulphide.
Substitution by alkynyl group (formation of alkynes)
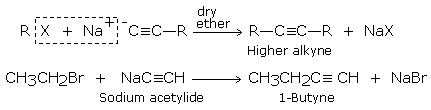
The formation of higher alkynes occurs when haloalkane is treated with sodium salt of alkynes (sodium alkynides): the halogen atom is replaced by alkyl group (-C C-).
The sodium alkynides needed for the above reaction are formed by the reaction of sodamide (or sodium in liquid NH3) with alkynes containing terminal triple bond.
 SureDen
SureDen