Named Reactions of Haloarenes
Sandmeyer's reaction;
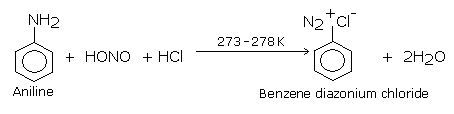
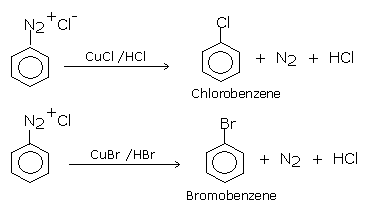
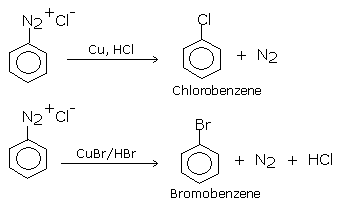
Aryl halides can be more satisfactorily obtained by the decomposition of aryl diazonium salts in the presence of copper halide solution dissolved in corresponding halogen acid , the diazo salt is replaced by a halogen atom.This reaction consists of treating freshly prepared diazonium salt solution with cuprous chloride or cuprous bromide. Chloro and bromoarenes are formed. Diazonium salts required for this purpose are prepared by treating ice-cold solution of aniline in excess of dilute HCl with an aqueous solution of sodium nitrite at low temperature (0-5oC). This reaction is known as diazotization reaction.
The benzene diazonium salt is used for preparing aryl halides as:
The Sandmeyer reaction has been modified to 'Gattermann reaction'.
In GATTERMANN REACTION:
the catalyst copper powder in yhe presence of corresponding halogen acid (HCL or HBr )
is used in the place of cuprous halide (cucl or CUBr).
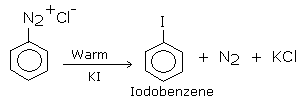
Warming the diazonium salt solution with aqueous KI solution by the above method gives iodoarenes. This is the best method for introducing iodine into benzene ring.
Balz-Schienmann reaction
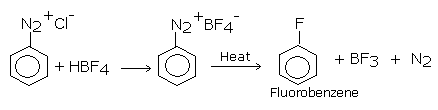
Aryl fluorides are prepared by the reaction of corresponding diazonium salt with fluoroboric acid .this reaction produces diazonium fluoroborate which on heating produces flourobenzene.
 SureDen
SureDen