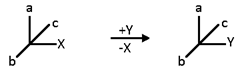Molecular Asymmetry
Molecular Asymmetry:-
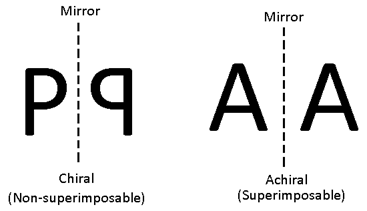
- A plane which divides an object into two symmetrical halves. Such that one half of the molecule is a mirror image of the other, is said to be the Plane of symmetry or mirror plane.
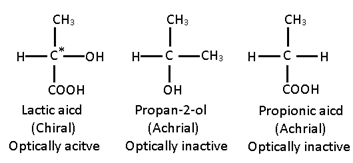
- An object having no plane of symmetry is called dissymmetric or chiral.
- A symmetric object is referred to as achiral. A dissymmetric or chiral object can be defined as the one that is not superimposable on its mirror image.
Enantiomers:-
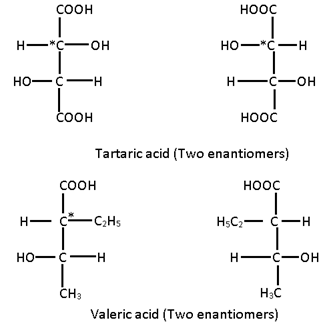
- Compounds which are mirror images of each other and are not supurimposable are termed enantiomers.
- The molecular dissymmetry or chirality is a necessary condition for the existence of enantiomers.
- A pair of enantiomers have identical physical and chemical properties but differ from each other in their action on plane polarized light in opposite directions but to the same extent.
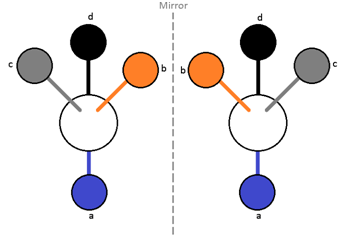
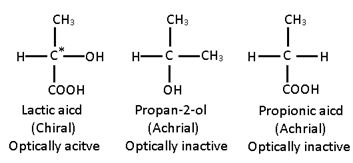
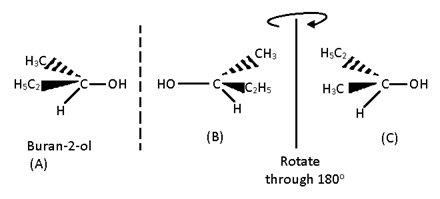
According to Van’t Hoff and Le Bel the four valencies of carbon atoms are directed towards the four corners of a regular tetrahedron. If there are four different atoms or groups a, b, c, d attached to four corners of a tetrahedron, then two different arrangements are possible which are not superimposable and bear the mirror – image relationship, i.e., act as enantiomers . Hence, a compound which consists of at least one asymmetric carbon atom is capable of showing the phenomenon of optical isomerism.
A Carbon atom which is bonded to four different atoms or groups in the molecule is called chiral carbon or tetrahedral centre or an asymmetric carbon atom (Chirality centre).
In Flying – Wedge representation, there types of lines are used in a standard way to indicate three dimensional structures in two – dimensional picture. A solid wedge, (think line) represents a bond projecting above the plane of the paper toward the observer. Continuous lines, - (solid lines) are bonds in the plane of the paper. A broken wedge, (dashed lines) is a bond below the plane (i.e., a bond pointing away from the observer).
The Racemization of one enantiomer (+ or -) or optically active compound into a racemic mixture (dl or ±) is termed as racemization.
Retention:
‘’the preservation of integrity of the spatial arrangement of bonds to an asymmetric centre during a chemical reaction.”
 SureDen
SureDen