Factors favoring SN1 and SN2 Mechanisms
Factors favoring SN1 AND SN2 Mechanisms:
Nature of alkyl halide
If the alkyl halide is primary, it reacts through SN2 and if it is tertiary, it reacts through SN1 mechanism. 2° Alkyl halide react through both mechanisms.
Nature of nucleophile
Strong nucleophiles favour SN2 mechanism whereas weak nuclephiles favour SN1mechanism.
Concentration of nucleophile
High concentration of nucleophile favours SN2, while low concentration favours SN1 mechanism.
Nature of solvent
Polar solvents favour SN1 mechanism as they can cause dissociation of alkyl halide and thus facilitate the formation of carbocation. Solvents of low polarity favour SN2 mechanism.
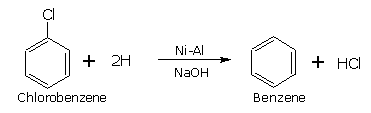
Reduction of chlorobenzene
With LiAlH4 or nickel aluminium alloy (Ni-Al), haloarenes undergo reduction to hydrocarbons in the presence of an alkali.
 SureDen
SureDen