Electrophilic substitution reactons of haloarenes
Electrophilic substitution reactons of haloarenes
Thus,
• Haloarenes undergo electrophilic substitution reactions slowly as compared to benzene.
• Halogen group is ortho and para directing (para-product usually predominates over the ortho product).
Ring electrophilic substitution reactions (directive influence of halogen for mono-substituted sompounds:
The halogen atom is slightly deactivating but artho and para directing and therefore, substitution takes place at o-and p-positions.
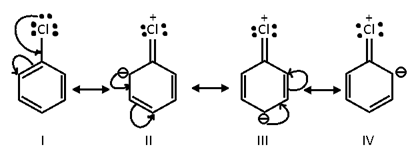
Resonating structures of chlorohenzene are given below:
The ortho and para directing effect of halogen atom can be understood, if we consider the resonance structures of chlorobenzene. In structures II, III and IV, there is a negative charge at o- and p- positions. In other words, because of resonance the electron density is relatively more at o- and p- positions than at m-position and hence the incoming eletrophile is more likely to attack on these positions resulting in the formation of o- and p- substituted products. However, because of steric hindrance at the o-postion, the p-product usually predominates over the o-product.
Further, the halogen atom has –I effect because it is an electron withdrawing group. As a result, the ring gets somewhat deactivated and so, haloarenes are less reactive than benzene towards electrophilic substitution reactions which occur slowly under drastic conditions.
 SureDen
SureDen