Steps of Metallurgy
- Crushing and Grinding of the ore:- the ores occur in nature as huge lumps. They are broken to small pieces with the help of crushers and grinders. The pieces are then reduced to fine powder in the stamp mill to give a pulverized mass.
- Concentration and benefaction of ore:- The ores are usually found mixed up with large amount of non-metallic impurities such as sand, mica, line stone and other earthly and rocky impurities. These unwanted impurities are called Gangue or Matrix and have to be removed before extraction the metals.
“The process of removal of unwanted impurities (gangue) from the ore and making it rich in the compound of metal is called ore concentration or ore Dressing or ore benefaction.”
Different methods are used to concentrate the ore depending upon the differences in physical properties of the metal is called and the impurities present in the ore.
Some of the methods of concentration of one are explained as under:
- Hydraulic Washing:-
This is based upon the difference in gravities of the ore and the gangue particles. It is therefore is also called gravity separation. In this method the powdered ore is heaped on a slant surface and powerful stream of water is forced into it. The lighter gangue particles are washed away and the heavier ore particles are left behind. This method is also called Levitation and is used for iron, gold, chromium etc.
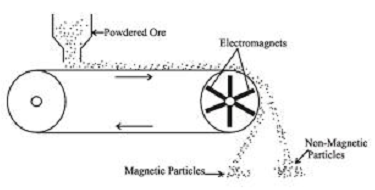
- Magnetic Separation:-
This method is based upon the differences in magnetic properties of the ore components. If etheir the gangue or the ore (one of these two) is attracted by a magnetic field, then the ore can be separated from the impurities with the help of magnetic separation method. In this method the crushed ore is dropped on a moving belt running on the wheals one of which is magnetic in nature (fig.). The magnetic portion of the ore is attracted by the magnetic roller and falls near to the roller, while the non magnetic impurities fall father off.
c. Froth Floatation Process:-
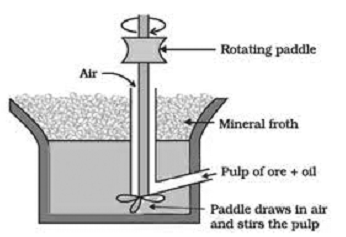 This method is widely used for the concentration of sulphide ores such as Zinc Blend (ZnS), copper pyrites (CuFeS2), Galena (PbS) etc. This method is based upon the fact that the surface of sulphide ores is preferentially wetted by oil while that of gangue is preferentially wetted by water.
This method is widely used for the concentration of sulphide ores such as Zinc Blend (ZnS), copper pyrites (CuFeS2), Galena (PbS) etc. This method is based upon the fact that the surface of sulphide ores is preferentially wetted by oil while that of gangue is preferentially wetted by water.
The ore is crushed into a fine powder and mixed with water to form a suspension in a tank. To it collectors and froth stabilizers are added. Collectors (e.g. pine oil, fatty acids, xanthates etc) enhance the non-wettability of the mineral particles and froth stabilizers (e.g. cresols, aniline) stabilize the froth.
When compressed air is passed and the mixture is agitated the mineral particles are wetted by oil while gangue particles by water. As a result froth is formed which carries the mineral particles. The froth being light floats over water and is skimmed off.
Sometime, to separate two sulphide ores depressants are used. e.g. in case of an ore containing ZnS and PbS, the depressant used is NaCN. It selectively prevents ZnS from coming to the froth but allows PbS to come with the froth.
(d) Leaching:-
This process consists in treating the powdered ore with a suitable reagent (such as acid, base or other chemicals) which can selectively dissolve the ore but not the impurities. It is widely used for Bauxite, Silver and Gold etc.
i) Leaching of Bauxite ore:- Bauxite ore consists of Fe2O3, SiO2 and TiO2 as impurities. The ore is digested with conc. solution of NaOH (45%) at 473-523 K. Under these conditions alumina (Al2O3) dissolves forming sodium meta aluminates and silica (SiO)2 dissolves as sodium silicate. The impurities Fe2O3, TiO2 remains undissolved and filtered off.
The solution is cooled and neutralized with CO2 when Aluminium hydroxide is precipitated. However some freshly precipitated aluminium hydroxide is also added to quicken the process. It is called seeding process.
The hydrated alumina, obtained above is filtered, washed and heated to 1473 K to get pure alumina.
It is known as Baeyer Process
ii.) Leaching of Silver and Gold:-
The process of leaching is also used for the concentration of ores of silver and gold by converting these metals or their ores into suitable complexes. In this process the finely powdered argentite (containing Ag2S) or native Silver or Gold is treated with a diluter solution (0.5%) of sodium or potassium cyanide. As a result of it, silver and gold pass into solution forming their respective soluble complex while impurities remain unaffected and are filtered off. Thus:
Soluble Complex
For silver:-
Na2S Thus formed is largely oxidized to Na2S by air
For Gold:-
Soluble Complex
3.Extraction of crude metal:-
The process used to obtain metals in free state from concentred ores is called extraction. It involves two chemical processes. (i) Conversion of ore into metallic oxide (ii)To obtain crude metal from concentrated ore.
- Conversion of ore into metallic oxide:- This method is also called the de-electronation of ore, as it involves the process of oxidation. Metals are usually present in ores as their hydrated oxide (hydroxide), carbonates and sulphides. Depending upon the nature of the minerals present in the ores, the following two methods are used for conversion of ores into their respective oxide.
- Calcination:- Calcination is the process of converting an ore into its oxide by heating it strongly below its melting point either in absence or limited supply of air.
This method is commonly used to convert metal carbonates and hydroxide to their respective oxides. During the process of calcinations, the following chemical change occurs (Uses of calcinations):
- Moisture is driven out
- Volatile impurities of S, As & P removed as their Volatile oxide
Water is removed from hydrated oxide and hydroxide ore:
Carbonate ores are converted into their respective oxides by the loss of carbon di-oxide:
(MALACHITE )
(CALAMINE)
It makes the ore porous and hence easily workable in subsequent stages. Calcinations is carried out in reverberatory furnace.
- Roasting:- Roasting is the process of converting an ore into its metallic oxide by heating strongly below melting point in excess of air. This process is commonly used for sulphide ores. During the process of roasting he following chemical changes occurs (uses of Rosting):
- Moisture is removed
- Organic matter is removed
- Non-metallic impurities like S, P and As are oxidized to their volatile oxides and remove.
Ores are generally converted into metallic oxides.
The SO2 thus produced is used for the manufacture of H2SO4.
If calcinated or roasted substance still contains earthy impurities an additional substance flux is usually added. Flux is a substance that chemically combines with gangue (Matrix) which may still be present in the roasted or the calcinated ore to form an easily fusible material called the slag.
Depending upon the nature of the impurities the flux can be classified as Acidic and basic flux. For Basic impurities such as lime or oxides of metals present in the ore acidic flux such as silica (SiO2) and borax (Na2B4O7.10H2O) etc. are used. e.g.
Impurity Acidic Flux Slag
For acidic impurities like sand (SiO2), Phosphorus pentaoxide (P4O10) etc. Present in ore, basic fluxes like lime stone (CaCO3), magnesite (MgCO3) are used.
Impurity Acidic Flux Slag
ii) To obtain Crude metal from Concentrated ore:-
This method is also called electronation as it involves reduction of the oxide ore. The roasted or calcinated ore is then reduced to metal by using a suitable reducing agent. The choice of the reducing agent however depends upon the reactivity of the metal. If the metal to be extracted is very reactive like the alkali metals (K, Na etc.), alkaline earth metals (Ca, Mg etc.) and aluminium, then the reduction of oxide can be done only by electrolytic method. The oxides of less reactive metals like such as Zinc, iron, copper, lead, tin, manganese, chromium etc. can be reduced by some suitable reducing agent such as carbon (coke), carbon monoxide or even another metal.
This process usually involves heating the metal oxide with a suitable reducing agent and hence is also called pyrometallurgy.
4.Refining of metal:-
A metal extracted by any method is usually contaminated with some impurity. For obtaining metals of high purity, several techniques are used, depending upon the differences in properties of the metal and the impurity. Some of them are listed as: (a) Distillation (b) Liquation (c) Electrolysis (d) Zone Refining (e) Vapour phase Refining (f) Chromatographic methods.
- Distillation:- This is useful for low boiling metals like zinc and Mercury. The impure metal is evaporated to obtain the pure metal as distillate.
- Liquation:- In this method a low melting metal like tin can be made to flow on a sloping surface. In this way it is separated from higher melting impurities, called dross. Lead and Bismuth are also separated by the same method.
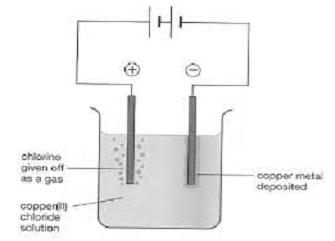
- Electrolysis:- In this method, the impure metal is made to act as anode. A strip of the same metal in pure form is used as cathode. They are put in a suitable electrolytic bath containing soluble salt of the same metal. The more basic metal remains in the solution and the less basic Anode mud. The reactions are:
Anode: M ⟹ Mn++ne
Cathode: Mn+ + ne ⟹ M picture
Copper is refined by this method. The electrolyte taken is CuSO4. Impurities from blister copper deposits as anode mud.
It contains antimony, selenium, silver, gold and platinum which settle as anode mud. These elements are less reactive hence are not affected by CuSO4 – H2SO4 solution and don not dissolve in electrolyte. Recovery of these metals may meet the cost of refining. Zinc may also be refined in the same way.
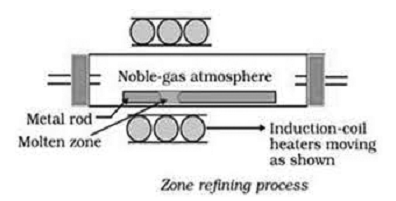
d) Zone Refining:- This method is based on the principle that the impurities are more soluble in the melt than in the solid state of the metal. A circular mobile heater is fixed at one end of a rod of impure metal. The molten zone moves along with the heater which is moved further. As the heater moves, the pure metal crystallized out of the melt and impurities pass on into the adjacent molten zone. The process is repeated several times. At one end, impurities are get concentrated. This end is cut off. This method is very useful for producing semiconductors and other metals of very high purity e.g.l germanium, silicon, boron, gallium and Indium.
e) Vapour Phase Refining:- In this method the metal is converted into its volatile compound and collected elsewhere. It is then decomposed to give pure metal. So, the two requirements are:
- The metal should form a volatile compound with an available reagent.
- The volatile compound should be easily decomposable, so that recovery is easy.
Nickel and Zirconium are refined by this method as:
Mond’s Process for Refining Nickel:- In this process, impure nickel is heated in a steam of carbon monoxide forming a volatile complex nickel tetra carbonyl:
The complex is subjected to higher temperature so that it is decomposed giving the pure metal.
Van Arkel Method for Refining Zirconium or Titanium:- The crude metal is heated in an evacuated vessel with Iodine, which forms covalent metal iodine and is volatile in nature.
The metal iodide is decomposed on a Tungsten filament, electrically heated to about 1800k. The pure metal is deposited on the filament.
- Chromatographic methods:-
This method is based on the principle that different components of a mixture are differently adsorbed on an adsorbent. The mobile phase and stationary phase are chosen such that components of the sample have different of the sample have different solubilities in the two phases. In one such method the column of Al2O3 (stationary phase) is prepared in a glass tube and the moving medium containing a solution of components (moving phase) is in liquid phase.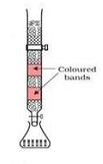
The mixture of components is put above the column. The component which is more adsorbed in the stationary phase takes longer time to travel through in the stationary phase takes longer time to travel through it than a component which is adsorbed less in stationary phase but very soluble in the mobile phase. This is an example of column chromatography.
This method is very useful for purification of the elements which are available in minute quantities and the impurities are not very different in chemical properties from the elements to be purified.
 SureDen
SureDen