Extraction of Iron
Extraction of Iron:-
Iron is usually extracted from the oxide ore i.e. hematite (Fe2O3). It involves the following steps:
- Concentration:- The ore on iron hematite is carried out by froth – floatation process.
- Calcination:- The concentrated ore is the calcinated i.e. heated strongly in the presence of a limited supply of air in a reverberatory furnace. During calcinations following charges occur:
- Moisture is removed
- Impurities of sulphur, phosphorus and arsenic escape as their volatile oxides SO2, P4O10, As2O5 respectively.
- Ferrous oxide (FeO) is oxidized to ferric oxide (Fe2O3) thereby prevention loss of iron as slag.
- The ore is made porous, hence is more suitable for smelting.
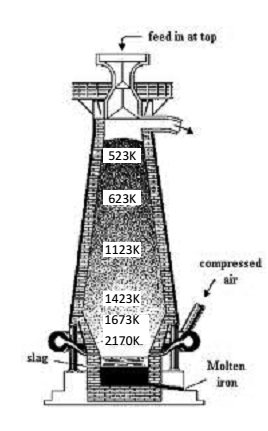
Smelting:- The calcinated ore is reduced with carbon i.e. smelted in a blast funace. As shown in figure. The charge consists of calcinated ore (8- parts), coke (4- parts) and lime stone (1 – parts) is introduced from the top of the furnace at the same time a blast of hot air preheated to 1000 K is blown into the furnace through tuveres. The following reactions occur in the blast furnace.
-
Zone of combustion:- Near tuyeres coke burns and the temperature is raised to 2170 K.
-
Zone of Absorption:- This is lower part of the furnace and the temperature here is 1423-1673 K. As CO2 formed near tuyeres moves up, it meets the descending charge. The coke present in the charge reduces CO2 to CO.
-
Zone of Slag Formation:- It is the Middle part of the furnace. The temperature here is around 1123K. In this region, limestone decomposes to form CaO and CO2. The CaO thus formed acts as flux and combines with silica (present as impurity) to form fusible calcium slag.
(slag)
-
Zone of Reduction:- This is the upper art of the furnace. The temperature here is around 823 K the ores are reduced to FeO by CO.
The further reduction of FeO to Fe by CO occurs around 1123K.
However direct reduction of iron ores, which might have escaped reduction around 823 K gets Completely reduced to iron by carbon above 1123K.
-
Zone of Fusion:- This is the lower part of the furnace. Temperature here is between 1423 – 1673 K. in this region, spongy iron melts and dissolves some C, S, P, Si, Mn etc. CaSiO3 slag also melts but forms upper layer being lighter in nature. The iron thus obtained has 4% carbon and may impurities like C, S, P, Si, and Mn. This is called Pig iron and is most impure form of iron.
Preparation of cast iron:- Cast iron is prepared from pig iron by melting pig iron with scrap iron and coke using hot air blast. It has lower C content (3%) and is extremely hard and brittle.
Preparation of Wrought Iron:- Wrought iron is the purest form of commercial iron having carbon content 0.2 – 0.5%. Wrought iron is prepared form cast iron by decreasing the carbon content and oxidizing the impurities (C, S, P, Si, Mn etc.) in a reverberatory furnace. On heating with haematite, C is oxidized to CO, S to SO2, Si to SiO2, P to P4O10 and Mn to MnO. CO and SO2 being gases are escaped to atmosphere and MnO, SiO2 and P4O10 are converted to slag.
(Slag)
 SureDen
SureDen