Nerst Equation
Nerst equation of electrode potential (Effect of electrolyte constrictions T)
Electrode potential is said to be standard electrode potential if measured under standard conditions of concentration l temperature. Electrolyte constriction = 1 m and t 298 K. But if constriction and T condition are not standard, then electrode potential comes out to different, obtained using Nerst equation. It deals with the value of reduction potential only.
Mn+ + ne- → M (s)
Concentration cell: - Cells in which two electrodes are of same material but the constriction of the electrolyte in then is different.
Ecell = (0.0591/n) log (C2 /C1)
Zn|Zn2+ (C1) ||Zn2+ (C2) |Zn
If C2 > C, EMF = +ve, Reaction takes place. But no net chemical change takes place.
Equilibrium constant for Nerst equation
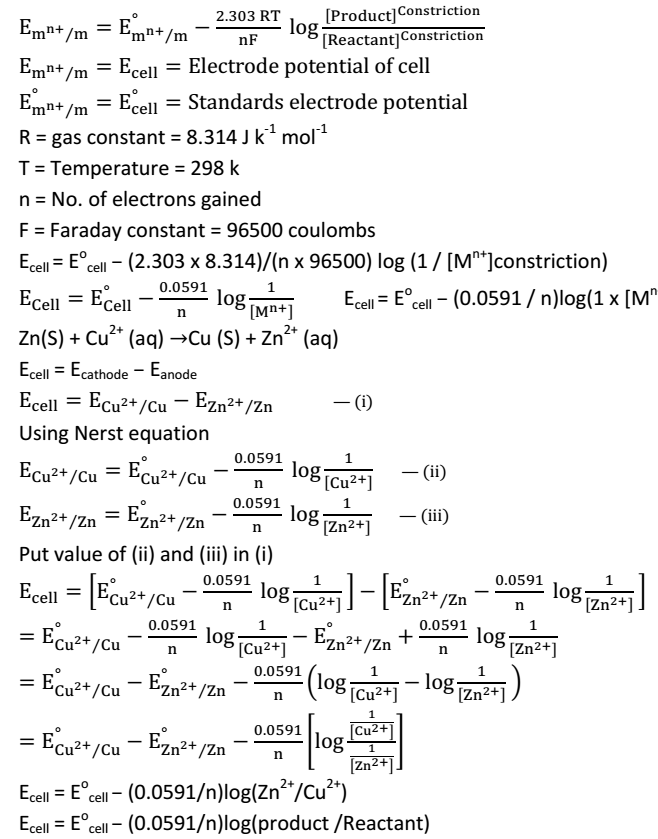
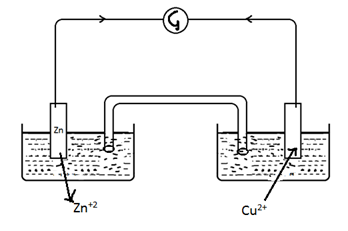
We take example of Zn(s) | ZnSO4 (aq) ||CuSO4 (aq) |Cu(s)
Same changes takes place are
- Zn from Zn rod passes into solution So constriction of ZnSo4 increases
- Cu from CuSo4 solution deposited on Cu rode constriction of CuSo4 decreases
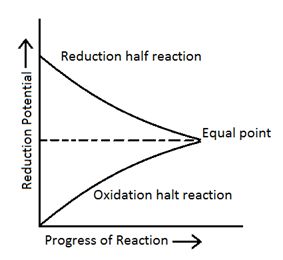
As electrode potential depends upon constriction of solutions, so electrode potential of both electrodes keep on changing as the constriction of both the solution being changing. Ultimately a stage comes when the electrode potential of 2 electrodes becomes equal and current stops flowing in circuit and EMF of cell becomes zero then cell reaction is said to be in equilibrium. So
Zn + CuSo4 → Cu + ZnSo4
[Zn2+]/ [Cu2+] = equilibrium = KC
When constriction = KC, then ECell = 0
0 = EoCell – (0.0591/n) log KC
EoCell – (0.0591/n) log KC
 SureDen
SureDen