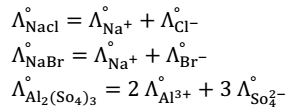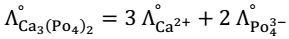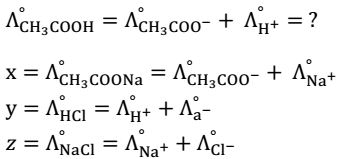Kohlrausch Law and its Applications
Kohlrausch law: - At the finite dilution, when dissociation of electrolyte is complete then every ion makes a definite contribution in the molar conductivity independent of the other ions present along with it in the solution.
Law can be defined as the molar conductivity of an electrolyte at infinite dilution is equal to the sum of limiting (at infinite dilution) ionic conductivities of cations and anions with each value multiplied by the no. of ions present in one formula unit of electrolyte.
Application of kohlrausch law:-
1. To calculate the molar conductivities of weak electrolyte at infinite dilution. E.g.-Acetic acid (CH3COOH)
The value of x, y, and z can be obtained from Debye Huckel on Sager Equation because they are strong electrolytes. To get we add (1) and (2) and subtract (3) equation
2. To calculate degree of dissociation and dissociation constant-
∝ = Amount dissociated / Total amount taken = ΛCm = ΛOm
ΛCm ∝ Amount dissociated
Λom ∝ Total amount taken (At infinite dilution all amount get dissociated)
From degree of dissociation (∝), dissociated constant (K)
Can be calculated = C∝2 / 1 - ∝
C = constriction or molarity
3. For calculation of solubility of sparingly soluble salt
Λom = (K x 1000) / M M=molarity
M = (K x 1000) / Λom
Strength (solubility) = molarity x molecular mass g/lit
 SureDen
SureDen