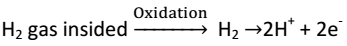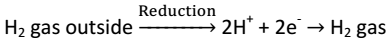Hydrogen Electrode and Electrode Potential
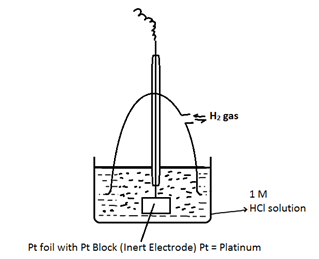
Construction and working of S.H.E or N.H.E
On S.H.E both oxidation as well as reduction can takes place
When S.H.E act as Anode, then
When S.H.E act as cathode, then
The electrode potential of S.H.E is taken as zero at 298 K
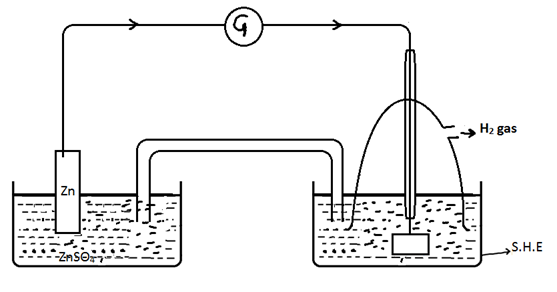
Measurement of electrode potential using S.H.E
|
Zn half cell |
S.H.E |
Zn is measured. Zn → Zn2+ + 2e- |
2H+ + 2e- → H2 gas |
|
|
Related Keywords
 SureDen
SureDen