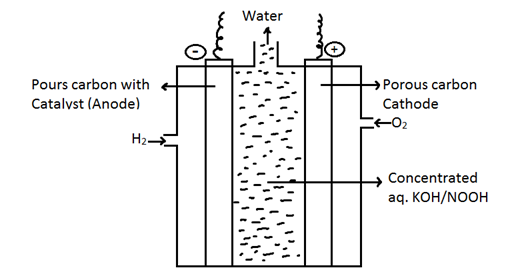
Fuel Cell
Fuel cells:- Cells which can convert energy produced during the confusion of fuels directly into electrical energy. Fuel can be hydrogen, methane, methanol etc catalyst used is platinum or palladium.
At Anode → 2H2 + 4OH- → 4H2O + 4e-
At Cathode → O2 + 2H2O + 4e- → 4OH-
----------------------------------------------------------
2H2 + O2 → 2H2O
This cell work continuously when fuel is supplied continuously Advantages of fuel cell.
1. Pollution free (only water is produced)
2. More efficient than other cells. Fuels cells are 60 – 70% efficient while others 35 - 45% efficient.
3. Can work at high temperature of up to 140°C.
4. Expected EMF is 1.23 V and actual is 0.9 V.
5. Superior to thermal power plant
6. First used in Apollo moon flight, water 200 kg of fuel had worked for 11 days and water produced is used for drinking purpose.
Difficulty in construction of fuel cells
1. Corrosiveness of electrolyte
2. Problem of handling gaseous fuels
3. High Cost of catalyst used
4. Providing the contact b/w 3 phases- Solid electrodes, liquid electrolyte, gaseous fuel.
 SureDen
SureDen