Electrolytic Conduction
Electrolytic Conduction: - Substance that allows passage of electricity is called conductors. Sub. That does’t allow passage of electricity are called insulators. Conductors are divided into 2 classes.
Metallic conductor
- Electronic conductor:- Which conduct electricity without undergoing any decomposition. e.g- Metals, graphite. In these conduction is due to flow of electrons.
Ionic conductor
- Electrolytic conductor:- Which conduct electricity and undergoes decomposition e.g- acid, bases and salts in water. In these conductor is due to flow of ions.
- Electrical Resistance:- Can be determined by ohm’s . Any sub. Cause hindrance or problem in flow of electric current, this problem is called Resistance. According to ohms law “At constant temperature, the voltage or electric potential (V) is directly proportional to current (I) opplied.
V x I
V = R x I
So resistance (R) = Voltage(V)/Current (I)
- Conductance :- The Reciprocal of electrical resistance is called conductance (C). C = 1/R
Units of resistance = ohm (Ω)
Units of conductance = ohm-1 or siemen or MhO
- Resistivity or specific resistance: - A constant depends material of conductor.
R ∝ l/A
R =ρ l/A
L = length of conductor
A = cross section Area
P = proportionality constant or resistivity or specific resistance.
Resistivity (ρ) = R x A /l = ohm x m2 / m = ohm. m = Ω. m
Units of resistivity are ohm. Meter (Ω.m)
-
Conductivity or specific conductance: - Reciprocal of resistivity or specific resistance. Denoted by kappa (k)
(k=1/ ρ)=l/(R x A) = (1/R) x (1l/A) = (C x l/A)
k=(c x 1)/ (A-->cell constant) If l = 1, A = 1 then
K = C
Specific conductivity can be defined as the conductance of a solution of 1 cm length and having 1 square cm area.
Units of conductivity = ohm-1 cm-1 or (Ω-1 cm-1)
- Equivalent conductivity: - Can be defined as the conductance off all the ions produced from 1 gram equivalent of electrolyte dissolved in any volume of solution represented by ΛEq.
ΛEq = K x v
If Solution has c gram equivalent per litre or 1000 cm3 then
ΛEq = Equivalent conductivity
K = Specific conductivity
v = vol. of Sol4 containing 1 gram equivalent.
ΛEq = K x 1000/C
ΛEq = K x 1000/normality
Units of ΛEq = k x v
=Ω-1 cm-1 x cm3/gm.Eq.
= Ω-1 cm2 Eq-1
- Molar conductivity:-
Defined as Constance produced by all the ions produced by 1 mole of electrolyte dissolved in any volume of Sol4
Λm = k x v = k x 1000/C = k x 1000/molarity
Units: - Ω-1 cm2 mol-1
Λm = k x 1000/m
= 5cm-1/mol = 5cm-1cm-3/mol
Cm3 = 5 mol-1 cm2
Measurement of electrolytic conductance, specific conductivity equivalent conductivity and molar conductivity.
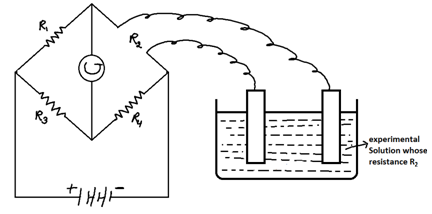
Electrolytic conductance: - Cab be measured by wheat stone bridge or Meter Bridge
We want to calculate the conductance or resistance of solution (R2). At null point, when defection in galvanometer is zero, then
R1/R2 = (R3/R4)R2 = (R1 x R4)/R3
Conductance (C) = 1/R2
In this experiment double distilled or conductivity water in used. It is pure water because it has no ions of it.
Conductivity (k) = 1/r = 1/R2 x l/a = conductance x cell constant
Variation of molar conductivity with concentration
- In case of strong electrolyte, molar conductance decreases with increase of dilution because strong electrolyte dissociate completely in solution so number of ions remains same even on dilution. But at higher concentration greater inter-ionic attraction retard the motion of ions so decreases
Conductivity (k) = No. of Ions/Volume
Dilution increases, constriction of ions increases inter ionic attract increases, v increases, k decreases so molar conductivity
(Λm) = (k x 1000)/M
If k decreases, then Λm also decreases
 SureDen
SureDen