Electrolysis
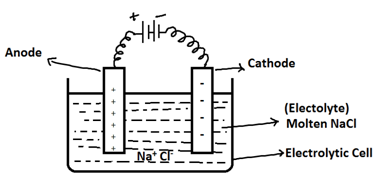
Electrolysis:- is defined as the process of decomposition of an electrolyte by case of electricity through it’s aqueous solution or molten state. Apparatus used for electrolysis is called electrolytic cell. It consists of electrolyte in aqueous/molten state and 2 metal electrodes, connected to a battery.
Mechanism:- Process of electrolysis follows the ionisation theory. According to this theory when every electrolyte is dissolved in water or melted, then electrolyte dissociates to produce +ve and –ve Ions, called cations and anions respectively, on passing electric current cations moves towards cathode (-ve electrode) and anions moves towards anode (+ve electrode) and become neutral. Oxidation occurs at anode while reduction occurs at cathode. Conversion of ions into neutral species is called primary change and new product formation after primary change is called secondary (2°) change.
Example of Electrolysis:- Electrolysis of molten sodium chloride (NaCl)
NaCl ⇌ Na+ + Cl-
At Cathode:- Na+ + e- --> Na (Primary change)
At Anode:- Cl- --> Cl + e- (Primary change)
Overall Reaction Cl + Cl --> Cl2 (Secondary change)
Or 2 NaCl
Electrolysis of molten NaCl Electrolyte takes place and provides the products Na and Cl2 gas at Cathode and Anode respectively.
Electrolysis of Aqueous sodium chloride: - in this NaCl is present along with H2O so called aqueous NaCl so NaCl and H2O both ionize as
NaCl --> Na+ + Cl-
H2O H+ + OH-
Now we have two cations Na+ and H+ and two anions Cl- and OH-, which can move towards cathode and anode respectively. But out of two only one can migrate towards respective electrode depending upon the discharge potential out of two which has lower discharge potential, will move towards respective electrode out of Na+ and H+, hydrogen ions has lower discharge potential so H+ ions will move towards cathode or –ve electrode and Na+ ions will remain in the solution out of Cl- and OH-, chloride ions have lower discharge potential so Cl- will move towards anode and produce Cl2 gas or secondary change.
At cathode: - H+ + e- --> H (1° change)
H + H --> H2 (2° change)
At anode: - Cl- --> Cl + e- (1° change)
Cl + Cl --> Cl2 (2° change)
Applications of Electrolysis
1. Production of hydrogen by electrolysis of H2O
2. Production of chlorine by electrolysis of NaCl
3. Extraction and purification of metals
4. Formation of heavy water (D2O)
5. Electroplating, electro refining, electrotyping
 SureDen
SureDen