Debye Huckel Onsager Equation
Debye Huckel Onsager Equation
ΛCm = ΛOm – b√c
ΛCm =Molar conductivity at any constriction C.
ΛCm =Molar conductivity at infinite constriction.
ΛOm =Molar conductivity at infinite constriction
b = Constant
(c)1/2 = concentration
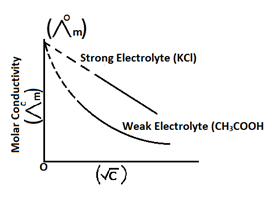
In case of weak electrolyte, conductance increases with increase in dilution, because dilution causes ionization of weak electrolyte. But it doesn’t reach the infinite or limiting value.
Limitation: - It cannot explain the molar conductivity of weak electrolyte at infinite dilution or limiting value. It is explained by kohlrausch law.
Related Keywords
 SureDen
SureDen