Commercial cells-Secondary
Secondary cells: - Those which can be recharged e.g. - Lead strong battery, NiCad cell
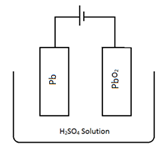
(1) Lead strong battery: - A 12 v lead strong battery is generally used which consists of 6 cells of 2v each. Each cell consists of a lead anode or a grid of lead filled with finally divided spongy lead. And a grid of lead packed with lead dioxide (P6O2) as cathode. Electrodes are separated by thin wooden or fibre glass sheet and suspended in dilute sulphuric acid. (38% by mass or having a density of 1.3 g cm-3, act as electrolyte). All compartments or cells are in series with each other.
At Anode →Pb → pb2+ + 2e-
Or
Pb + So42- → PbSo4 + 2e-
At cathode → Pbo2 + 4H+ + So42- → PbSo4 + 2H2o + 2e-
-----------------------------------------------------------------------------------
Pb + Pbo2 + 4H+ + 2So42- → 2PbSo4 + 2H2o
The PbSo4 produced, sticks to the respective electrodes and density of H2So4 fall to 1.2 g cm-3. when density is below 1.2 g cm-3, the cell needs recharging using external source of current. Then reaction will be reversed. Then reactant changed to product and product into reactants.
At cathode → PbSo4 + 2e- → Pb + So42-
At anode → PbSo4 + 2H2o → Pbo2 + So42- + 4H+ + 2e-
-----------------------------------------------------------------------------
2PbSo4 + 2H2o → Pb + Pbo2 + 4H+ + 2e
Water was to be added periodically because during recharging some water is lost due to its electrolysis to H2 and O2
In maintenance free batteries, an alloy of Ca & Pb is used which prevents electrolysis of water
(2) Nickel-cadmium battery (NiCad battery):- More expensive but has longer like due to less corrosive nature of electrolyte.
Anode → Cd + 2OH- → Cd (OH)2 + 2 e-
Cathode → NiO2 + 2H2O + 2e- → Ni(OH)2 + 2OH-
----------------------------------------------------------------------------
Cd + NiO2 + 2H2O + 2H2O → Cd (OH)2 + Ni(OH)2
Electrolyte used in KOH or NaOH solution
During recharging
Ca(OH)2 + 2e- → Cd + 2OH-
Ni(OH)2 + 2(OH)- → NiO2 + 2H2O + 2e-
------------------------------------------------------------------
Cd(OH)2 + Ni(OH)2 → Cd + NiO2 + 2H2O
As no gases are produced during discharging or recharging so battery can be sealed used in computers, laptop, calculators etc.
 SureDen
SureDen