Commercial cells-Primary
Commercial cells
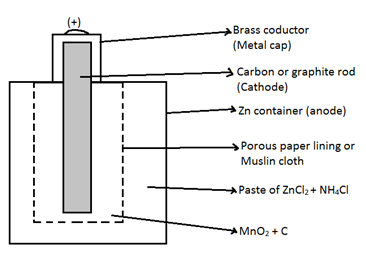
1. Primary cell: - Those in which the reaction taking place cannot be reversed. So cell can’t be recharged. e.g. - (1) Dry cell
Oxidation at Anode → Zn → Zn2+ + 2e-
Reduction at cathode → 2MnO2 + 2NH4+ + 2e- → Mn2O3 + 2NH3 +H2O
![]() Overall reaction → Zn + 2MnO2 + 2NH4+ → Mn2O3 + 2NH3 + H2O + Zn2+
Overall reaction → Zn + 2MnO2 + 2NH4+ → Mn2O3 + 2NH3 + H2O + Zn2+
The Ammonia (NH3) evolved combine immediately with ZnCl2 to form a complex-
ZnCl2 + 2NH3 → [Zn (NH3)2 Cl]
Hence cell will not burst, due to NH3
As the overall reaction involves ions so the cell can’t provide a constant EMF. Because with time Zn+2 and NH4+ decreases The EMF ranges from 1.25 to 1.50 V
The ammonium chloride (NH4Cl) used is corrosive because it produces HCl which corrodes the Zn metal and there is change of leakage of cell.
NH4Cl + H2O → NH4OH + HCl
Paste include H2O, due to which HCl produced will make the container to leak
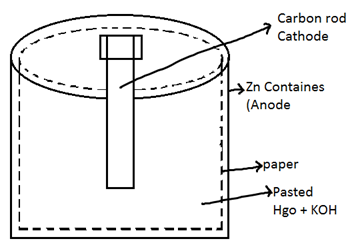
(2) Mercury cell (Ruben – Mallory cell) or Button cell: - used in devices which require low current.
At Anode
Zn → Zn2+ + 2e-
2 Zn + 4 OH- → 2 ZnO + 2 H2O + 4e-
At cathode
![]() 2 HgO + 4 e- + 2 H2O → 2 Hg + 4 OH-
2 HgO + 4 e- + 2 H2O → 2 Hg + 4 OH-
2 Zn + 2 HgO → 2 ZnO + 2 Hg
Or
Zn + HgO → Hg + ZnO
As the overall reaction is not involving any kind of ions so it can provide a constant EMF of 1.35 volt.
 SureDen
SureDen