Organo Metallics
Organo-metallics:-
Bond between metal and carbon of organic molecule.
Sodium ethoxide is not organo metallic as sodium is linked with oxygen.
- σ–Bonded:-
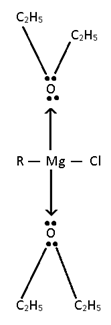
Metal is directly attached with carbon using –bonded.
e.g:- CH3 – Li, (C2H5)4 Pb, R3Al, R-MgX
2..π – Bonded:-
π-Bond between two atoms is co-ordinated towards vacant d-orbital of metal by using delocalization.
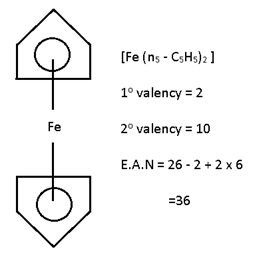
eg:- Ferrocene
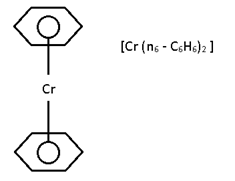
Bis (Benzene Chromium)
3..
- σ and π Both Bond:-
contain vacant anti-bonding molecular orbitals. Therefore they can show synergic effect.
These legands donate their lone pair as sigma bond towards vacant d-orbital of metal and filled d electrons of metals are delocalized in anti-bonding -orbitals of legands. Such legand -donor and -acceptor legand -acid legand. Therefore they form most stable complexes.
CO is best -acceptor and CN- is best -donor and poor -acceptor compared to CO.
- [Ni (CO)4 ] ------==> Sp3
- [Fe (CO)5 ] -----==> dSp3
- [Cr (CO)6 ] ------==> d2Sp3
Since carbonyl accept electron in its anti-bonding orbital. Its bond order is slightly decreased. And bond length is slightly increased.
- Strongest metal carbon is present in:-
- [Ni (CO)4 ]
- [Cr (CO)6 ]
- [Mn (CO)6 ]+
- [V (CO)6 ]-
Ans:- 4) [V (CO)6 ]-
 SureDen
SureDen