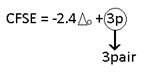Order of Energy in Octahedral
Order of energy in octahedral:-
dx2 – y2 = dz2 > dxy = dyz = dzx
d1, d2, d3 do not care that whether the legand is weak or strong.
|
Weak Legand |
Strong Legand |
|||||||
|
High spin energy |
Low spin Energy |
|||||||
|
d-orbital |
t2g |
eg |
Spin multiplicity |
CFSE |
t2g |
eg |
Spin multiplicity |
CFSE |
|
d1 |
1 |
0 |
+1/2 |
-0.4 Δo |
1 |
0 |
+1/2 |
-0.4 |
|
d2 |
2 |
0 |
+1 |
-4.08Δo |
2 |
0 |
+1 |
-0.8 |
|
d3 |
3 |
0 |
+3/2 |
-1.2Δo |
3 |
0 |
+3/2 |
-1.2 |
|
d4 |
3 |
1 |
+2 |
-0.6Δo |
4 |
0 |
+1 |
-1.6 |
|
d5 |
3 |
2 |
+5/2 |
Zero |
5 |
0 |
+1/2 |
-2.0 |
|
d6 |
4 |
2 |
+2 |
-0.4Δo |
6 |
0 |
Zero |
-2.4 (max. value most stable) |
|
d7 |
5 |
2 |
+3/2 |
-0.8Δo |
6 |
1 |
+1/2 |
-1.8 |
|
d8 |
6 |
2 |
+1 |
-1.2 |
6 |
2 |
+1 |
-1.2 |
|
d9 |
6 |
3 |
+1/2 |
-0.6 |
6 |
3 |
+1/2 |
-0.6 |
|
d10 |
6 |
4 |
Zero |
Zero |
6 |
4 |
Zero |
Zero |
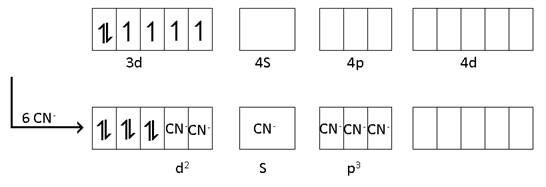
d2sp3, diamagnetic, inner, low spin
μ = 0 Configuration = t26g eg0
Configuration is written for metal only not for the legand as bond is Ionic not covalent.
Fe = +2 (Oxidation state)
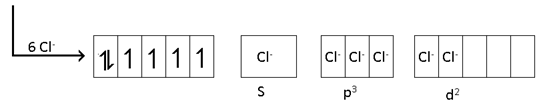
[FeCl6]-4 ==>Sp3d2 , pare, high spin
Configuration:-
t2g4 eg2
C.F.S.E ==> -0.4 Δo + P
∆O = (-4x + 6y) Dq
-
[Fe (CN)6 ]-3 d2Sp3, para, inner, low spin.
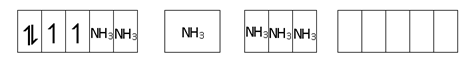
- Cr (NH3)6 ]+3 Cr + 3 = 3d3
d2sp3, Para,
t2g3 eg0
CFSE = -1.2 Δo
 SureDen
SureDen