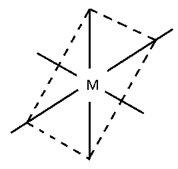
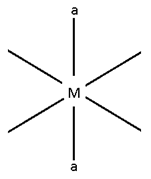
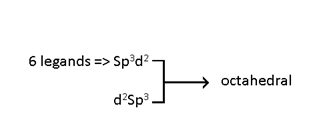
Octahedral complexes
Octahedral Complexes:-
Possibilities:-
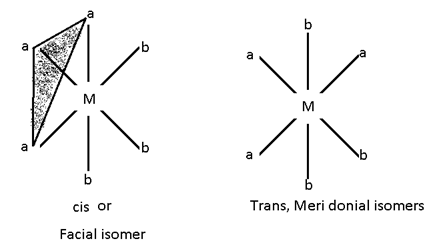
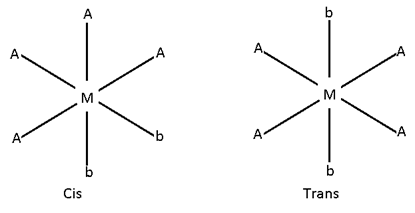
Ma3b3 :- [Co (NH3)3 Cl3 ]
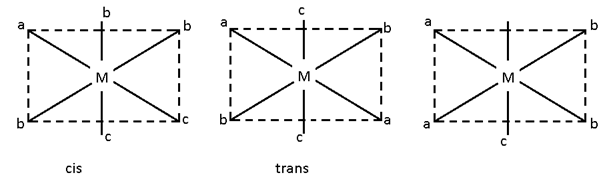
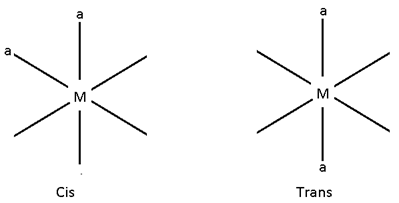
Ma2b2C2
In this case there are 5 possibilities:-
- All the legands are at 90°.
- All at 180°
- a – a, b, b = 90°, c – c = 180°
- a – a, c – c = 90, b – b = 180
- b – b, c – c = 90° and a – a = 180°
Ma2bcde
- M (AA)3
No geometry
e.g.:- [ G (en)3 ] Cl3
- M (AA)2b2
[ Pt (en)2 Cl2 ]
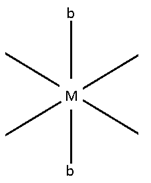
- M (AA)b4
b are fixed and
AA is at 90° and are fired
No geometry exists.
- M (AA)b2 C2
[ Pt (en)Cl2 Be2 ]
Cases:-
- B – B = 90° C – C = 90°
B – B = 90° C – C = 180°
B – B = 180° C – C = 90°
- Mabcd
No geometry
[Zn (NH3) (H2O) (Cl) (Br)]
Related Keywords
 SureDen
SureDen