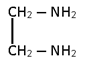Isomerism
Isomerism:-
Complexes having some molecular formula but different structure are called structural Isomer and in such Insomer 3 – D Orientation is not considered.
Ionisation Isomerism:-
Some formula, but diff structure due to different ion in aqueous medium.
e.g.:- [Cr (NH3)5 Cl] SO4 à [ ]+2 + SO42-
Hydrate Isomerism:-
Some molecular formula but different structure due to different no. of water molecule as legands or in crystallization.
Linkage Isomerism:-
This Isomerism is observed in ambidentate legand. Some formula but different structure due to different donor atom of same legand.
[Pt (H2O)4 (Cl) (NO2)] Br2 . 2H2O
[Pt (H2O)3 (ONO) (Br)2] Cl . 3H2O
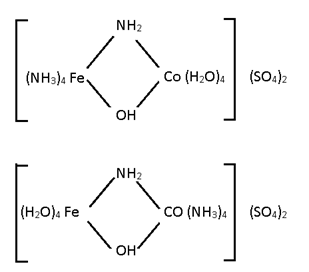
Co-ordination Isomerism:-
(When both cation and anion complexes are present).
Some metal with different legand
[Ni (NH3)4 ] [CuF4]
[ Cu (NH3)4 ] [NiF4]
Co – ordination position Isomer:-
Different molecular formula due to different position of legand in same complex. It is observed in Bridge complexes.
Polymerization Isomerism:-
Some empirical formula but different molecular form.
[ Pt (NH3)2 Cl2 ] and
[Pt (NH3)4 ] [PtCl4]
Legand Isomerism:-
Some molecular formula of legand with same donor but at different position.
Stereo Isomers:-
Plane:-
When divided in two equal part face parallel part.
Can divide in 3 parts,
2 vertical + 1 horizontal
Complexes having same molecular formula same structure but different orientation of legands in the 3D space are called Stereo Isomerism.
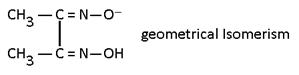
Geometrical Isomerism:-
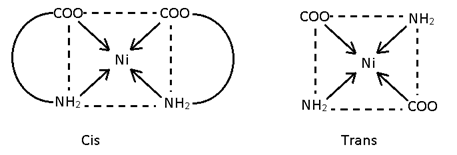
This isomerism is possible in square plane & Octohedral complexes because in such complexes distance between legands can be changed it is not possible in tetrahedral complexes because distance between legand is always same. If same legand are only at 90° complex is Cis and if 180° or 90°, 180° then both complexes is trans.
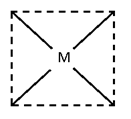
Square Planer Complex:-
- Ma4 Ma3b No geometry.
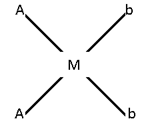
- Ma2b2
e.g.:- [Pt (NH3)2 Cl2]
a, b, c, d, e, f, ---------------==> unidentate legands.
AA ==> Symmetrical bidentate in
AB, AA’ ==> Unsymmetrical bidentate legand
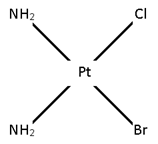
Ma2bc
[Pt (NH3)2 Cl Br]
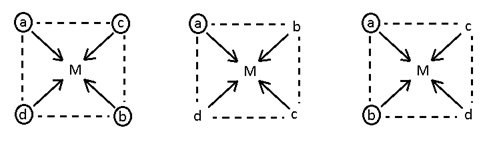
- Mabcd (3 geometrical isomerism)
[Pt (NH3) (H2O) (Br) (Cl)]
- M(AA)2 ==> [Pt (en)2 ]Cl2
No geometrical Isomerism
M (AA’)2, M(AB)2
B)
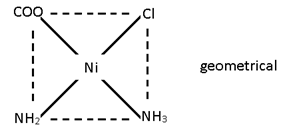
M(AA)b2
C)
M (AA’)b2
[Ni (gly) (NH4) Cl]
 SureDen
SureDen