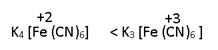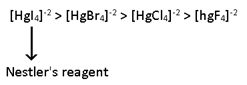Application of Crystal Field Theory
What is Crystal Field Theory?
Crystal Field Theory (CFT) is a model used to explain the electronic structure of complex metal ions in crystals. It describes how the electrostatic interaction between the metal ion and the surrounding ligand (non-metal) ions causes a splitting of the metal ion's d- or f-orbitals. The theory is used to predict the colors and magnetic properties of coordination compounds, which are compounds that contain metal ions bonded to ligands. CFT is a relatively simple model that is able to explain many of the observed properties of coordination compounds, but it has limitations and is not always able to accurately predict the properties of certain compounds.
CFT is based on the idea that the metal ion in a coordination compound is surrounded by a certain number of ligand ions, which are negatively charged. The electrostatic interaction between the positive metal ion and the negative ligands causes a splitting of the metal ion's d- or f-orbitals. This splitting is known as the crystal field splitting energy (CFSE). The magnitude of the CFSE depends on the spatial arrangement of the ligands around the metal ion, as well as on the nature of the ligands themselves.
The crystal field theory is able to explain why some metal ions are colored and others are not. The theory predicts that if the CFSE is large enough, it will cause the d- or f-orbitals to split into different energy levels. Electrons in the highest occupied energy level will be able to absorb light of a specific frequency, which corresponds to a specific color. The theory also predicts that if the CFSE is too small, the d- or f-orbitals will not split, and the metal ion will not be colored.
CFT is able to explain many of the observed properties of coordination compounds, such as the colors of complex ions and the magnetic properties of coordination compounds. The colors of coordination compounds are due to the absorption of light by the d- or f-electrons in the metal ion. The magnetic properties of coordination compounds are due to the presence of unpaired electrons in the d- or f-orbitals.
However, CFT is not able to accurately predict the properties of certain compounds. The theory does not take into account the interactions between the electrons in the metal ion and the ligands, which can have a significant effect on the properties of the compound. Additionally, the theory assumes a symmetric arrangement of ligands, which is not always the case in real compounds. Despite its limitations, CFT is a useful model for understanding the electronic structure of coordination compounds and it is widely used in inorganic chemistry.
Crystal Field Theory (CFT) has several applications in inorganic chemistry, including:
-
Predicting the colors of coordination compounds: CFT is able to explain the colors of coordination compounds, which are due to the absorption of light by the d- or f-electrons in the metal ion. The theory predicts that the energy levels of the d- or f-orbitals will split into different levels, with the highest energy level being designated as the "crystal field splitting energy" (Δ). The magnitude of Δ is determined by the charge and the spatial arrangement of the ligands.
-
Understanding the magnetic properties of coordination compounds: CFT also helps to explain the magnetic properties of coordination compounds, which are due to the presence of unpaired electrons in the d- or f-orbitals. The theory predicts that the electrons in the d- or f-orbitals will occupy the lower energy levels, which can lead to the presence of unpaired electrons and hence magnetic moment.
-
Determining the stability of coordination compounds: CFT also helps to predict the stability of coordination compounds. It predicts that the higher the crystal field splitting energy (Δ) is, the more stable the compound is.
-
Understanding the reactivity of coordination compounds: CFT can also be used to explain the reactivity of coordination compounds. It predicts that the compound with the lowest crystal field splitting energy is more likely to react than the compound with the highest crystal field splitting energy.
-
Designing new coordination compounds: CFT can be used to design new coordination compounds by predicting the properties of a compound before it is synthesized.
-
Analyzing spectroscopic data: CFT can be used to analyze spectroscopic data to determine the crystal field splitting energy of a coordination compound.
Application:-
- This theory can describe stability of complexes:-
More will be CFSE, more will be stability.
In case of different period matals, it 50% complexes are more stable and 3D are least.
[Pt (NH3)6 ]+2 > Pd (NH3)6 ]+2 > Ni (NH3)6 ]+2
2.For same D-series, high will be valency of central atom, more will be CFSE (due to more attraction)
Therefore more will be stability
3. For same metal, same valency, stronger will be legand, more be stability.
This shows carbonyl and cyanide are most stable after macrocycles and chelates and at last normal complexes are arranged.
[Cr (CO)6 ] > [Cr (CN)6 ]-3 > [Cr (en)3 ]+3 > [Cr (NH3)6 ]+3 > [Cr (H2O)6 ]+2 > [Crcl6 ]-3
- Oxalate is very weak legand but if 3 oxalate legands are present it behaves like strong legand and can make pairing.
[ Co (C2O4)3 ]+3 > [Co (H2O)6 ]+3
(Due to chelating agent)
Exception:-
Stability of Halide complexes
⟹ I- > Br- > Cl- > F-
4..For same D-series, same valency, same legand, smaller will be size of metal cation. More will be its electro negativity and more will be stability of complex.
It is called Irwin William order.
Mn2+ < Fe+2 < Ca+2 < Cu+2
-----------==> Stability
[Cu (NH3)4 ]+2 > [Ni (NH3)4 ]+2
More will be CFSE, higher will be value of formation constant | stability constant and more will be stability of complex.
What are the Limitations of CFT?
-
It assumes a symmetric arrangement of ligands: CFT assumes that the metal ion is surrounded by a symmetric arrangement of ligands, which is not always the case in real compounds. This can lead to inaccuracies in the predicted properties of the compound.
-
It does not take into account the interactions between the electrons in the metal ion and the ligands: CFT only considers the electrostatic interactions between the metal ion and the ligands, but the interactions between the electrons in the metal ion and the ligands can have a significant effect on the properties of the compound.
-
It does not account for the effect of the electron-electron repulsion between the metal ion's electrons: CFT only considers the electrostatic interactions between the metal ion and the ligands, but the electron-electron repulsion between the metal ion's electrons can play a significant role in the electronic structure of the compound.
-
It does not account for the effect of the metal-ligand hybridization: CFT does not consider the hybridization of the metal and ligand orbitals, which can affect the electronic structure of the compound.
-
It does not account for the effect of the metal-ligand bonding: CFT only considers the electrostatic interactions between the metal ion and the ligands, but the nature of the metal-ligand bonding can also affect the electronic structure of the compound.
Despite these limitations, CFT is a useful model for understanding the electronic structure of coordination compounds and predicting their properties. In many cases, it is able to provide a good qualitative explanation of the observed properties of coordination compounds, but it is not always able to accurately predict the properties of certain compounds.
 SureDen
SureDen