Cleansing Agents
Cleansing Agents:-Soaps and Detergents are mainly used.
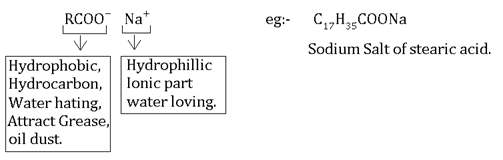
Soaps:-These are the sodium or potassium salts of long chain fatty acids e.g. palmitic acid, stearic acid.
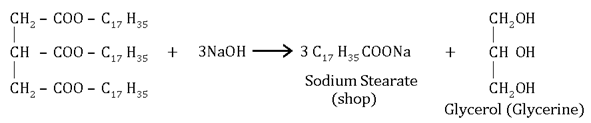
Saponi fication:-The formarion of soap or sodium extra of long chain fatty acid by heating fat in aqueous NaoH.
Types of Soaps
- Toilet Soap:- Prepared by using better grades of fat & oil. In these excess alkali are removed. These are made more attractive by adding colour & perfumes.
- Transparent Soap:- Made by dissolving soap in ethanol.
- Medicated Soap:- Some medicinal value is added in soap.
- Shaving Soap:- Contain glycerol to prevent rapid drying.
- Laundry Soap:- Contain filters like sodium silicate etc.
Question:- Why do Soaps do not work in hard water?
Answer:-Hard water contains calcium and Magnesium salts
In these Ca & Mg form insoluble soap & they do not work.
Synthetic Detergents:-These are the salts of aromatic benzene sulphonates, that work in both soft as well as ward water.
Synthetic Detergents are classified into 3 categories
- Anionic Detergents:- These are the sodium salts of sulphonated long chain alcohols or hydrocarbons. In these anionic part of detergent participate. In cleansing action.
e.g. CH3(CH2)10 CH2 oSo3- Na+ Used in toothpaste also.
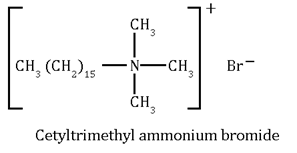
2. Cationic Detergents:- These are quaternary ammonium salts of amines with acetates, halides as anion. e.g.
3. Non-Ionic Detergents:- They do not contain any ions. e.g. CH3(CH2)16(CH2)4 CH2CH2OH
 SureDen
SureDen