Methods to predict the Rate Law
Methods to predict the rate law
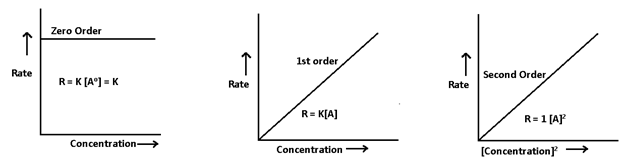
1. Graphical method or differential rate law method:- From the given data of concentration of reactant at different time intervals, the rate of reaction is calculated and then rate is plotted versus concentration
2. Initial rate method:- The same reactant is carried out by taking different initial concentration of the reactant and every time initial rate is calculated.
Question:- For the reaction 2 A + B → C + D
|
Exp. No. |
Initial concentration (A°) |
Initial concentration (B°) |
Initial rate of formation of D |
|
1 |
0.1 |
0.1 |
7.5 x 10-3 |
|
2 |
0.3 |
0.2 |
9.0 x 10-2 |
|
3 |
0.3 |
0.4 |
3.6 x 10-1 |
|
4 |
0.4 |
0.1 |
3.0 x 10-2 |
(i) Predict the order of reaction with respect to A and B and add the overall order.
(ii) Calculate the rate of formation of D when concentration of A equal to 0.8 mol/l and concentration of B 0.5 mol/l.
Answer:- (i) Let order w.r.t. A = ∝
Let order w.r.t. B = β
So Rate of formation of D = K [A]∝ [B]β
7.5 x 10-3 = k [0.1] ∝ [0.1] β — (i)
9.0 x 10-2 = k [0.3] ∝ [0.2] β — (ii)
3.6 x 10-1 = k [0.3] ∝ [0.4] β — (iii)
3.0 x 10-2 = k [0.4] ∝ [0.1] β —(iv)
Divide (i) by (iv)
∝ = 1
Divide (ii) by (iii)
β = 2 so overall order of reaction = ∝ + β = 1 + 2 = 3
(ii) Rate = k [A]∝ [B]β
Rate of reaction = 7.5 x [0.8]1 [0.5]2 = 1.5 mol L-1 sec-1
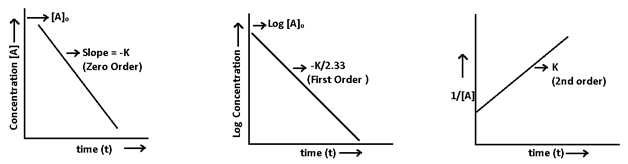
3. Integrated rate law method:- It cab be used in two different ways – graphical and substitution method.
- Graphical method:-
- Substitution method:- In this value of rate constant is put into formulas of different order. The expression or formula which gives the constant value of rate constant (k) will decides the order of reaction.
But this method has a limitation that this is a hit & trial method.
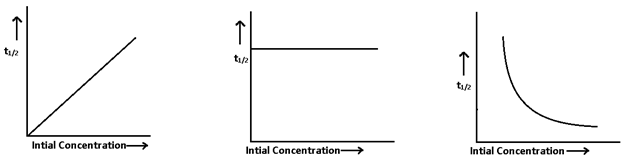
4. Half life:- Two half life a reaction depends upon the initial concentration of reactant so t1/2 ∝ (1/[A]n-1) or [Initial concentration]1-order
This can also be calculated by two methods-graphical and substitution method.
- Graphical method:- In this we draw graph b/w half life and initial concentration of reactant.
If t1/2 ∝ [Initial concentration], then zero order reaction
If t1/2 Independent initial concentration, then first order reaction
If t1/2 ∝ 1/[Initial concentration], then second order reaction
- Substitution method:- In this we have 2 different initial concentration. So their half life will be
[t1/2]1 = k [A°]11-order —(i)
[t1/2]1 = k [A°]21-order —(ii)
Divide (ii) by (i)
Taking log both side, then
5. Ostwald isolation method:- It is used for those reaction which involve more than one reactant. e.g.
A + B + C → Products
Rate = k [A]∝ [B]β [C]È£
In this method concentration of all the reactant is taken in large excess except one. Then the order is predicted for the reactant taken in small amount.
In second experiment, another reactant is taken in small amount and effect of it’s concentration are studied on the rate of reaction.
 SureDen
SureDen