Effect of catalyst
Effect of catalyst on the rate of reaction
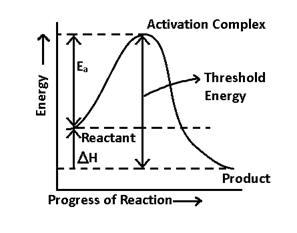
A catalyst is a substance which increase the speed or rate of reaction by providing an alternative pathway, involving less activation requirement
When we provide catalyst, activation energy requirement is less.
Characters tics of a catalyst & catalyzed reaction
1. Small amount of catalyst is sufficient to bring large change in rate of reaction.
2. Catalyst can’t initiate, it only accelerate a reaction.
3. It doesn’t change the free energy change (ΔG)
4. It doesn’t alter the enthalpy (ΔH) of a reaction.
5. Catalyst is regenerated after completion of reaction.
6. Catalyst doesn’t disturb the equilibrium.
Related Keywords
 SureDen
SureDen