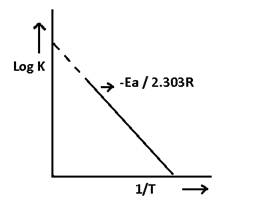
Arrhenius equation
Arrhenius equation:- It shows the effect of temperature on rate of reaction and on rate constant. It can be written as-
K = A e- Ea/RT — (i)
A = constant or collision frequency or Pre-exponential factor
Ea = Activation energy
R = Gas constant (8.314 J k-1 mol)
T = Temperature
e = Exponential
A and Ea, Collectively are called Arrhenius parameters.
Take log on both side of equation
ln k = ln A – Ea/RT — (ii)
At T1, rate constant is K1 and at T2, rate constant is K2
ln K1 = ln A – (Ea/RT1) --------(iii)
ln K2 = ln A – (Ea/RT2) --------(iv)
Subtracting the equation (iii) from (iv)
Related Keywords
 SureDen
SureDen