Monosaccharides
(1) MONOSACCHARIDES
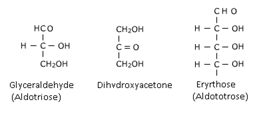
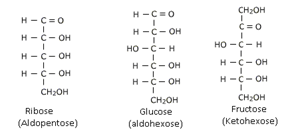
These are the simple carbohydrates which cannot be hydrolyzed to still simpler carbohydrates. They may be aldoses or ketoses depending upon the presence of aldehyde or ketone groups. They are also classified as trioses, tetroses, pentoses, hexoses etc depending upon the total no. of carbon atoms present. For example
Glucose and Fructose are common examples.
There are a number of chirality centres hence they possess optical isomers. The number of optical isomers is 2n (n = chirality centre). Conventionally a monosaccharide is given D – configuration if –OH group attached to the carbon adjacent to –CH2OH group is on right hand side and L – configuration if –OH group is on left hand side to the carbon adjescent to –CH2OH group.
1. Inspite of the presence of an aldehydic group glucose does not restore the pink colour of Schiff’s reagent and does not form addition in glucose is not free.
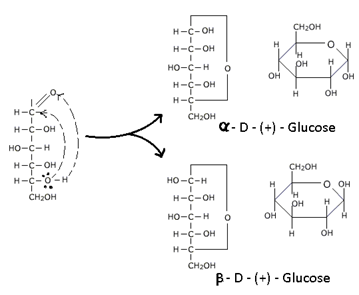
2. D – (+) – Glucose exists in two isomeric forms α –D – (+) – Glucose having melting point 146°C and specific rotation + 111° and β – D - (+) – Glucose having melting point 150°C and specific rotation + 19.2°.
When either of the α – or β – forms of glucose is dissolved in water and allowed to stand the specific rotation changes slowly and attains an equilibrium value of + 52.7° represented as:
α – D – (+) – Glucose Equilibrium Mixture
β – D – (+) – Glucose
[α]D = +111° [α]D = +52.7° [α]D = +19.2°
This spontaneous change in specific rotation of an optically active compound with time, to an equilibrium value, is called “Mutarotation”.
The above facts can be explained in terms of cyclic structure of glucose. The cyclic structure of glucose is formed by hemiacetal formation of –OH group of C5 and –CHO group, to give a ring structure of five-carbons and one oxygen atom. This structure resembles with Pyran
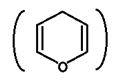 hence is called Pyranose structure.
hence is called Pyranose structure.
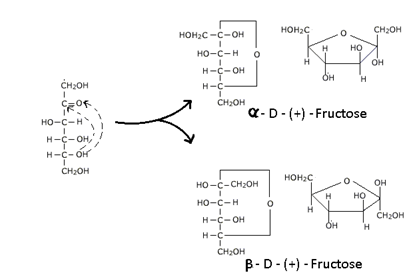
Similarly Fructose can also be represented in terms of five membered ring structure resembling Furan 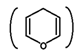 hence is called Furanose structure. It forms Hemiketal with –OH group of C5 and carbonyl group of C2.
hence is called Furanose structure. It forms Hemiketal with –OH group of C5 and carbonyl group of C2.
In the ring structure of Glucose and Fructose the α – and β – forms are associated with the position of –OH at the carbonyl carbon atom. In α – form –OH group is to the right and in β – form –OH group is to the left. “Such pair of steroisomer which differs in configuration only around the carbonyl carbon (C1 for Glucose & C2 for fructose) is called Anomers and the carbon atom is called Amoneric Carbon or Glycosidic Carbon.”
 SureDen
SureDen